About Authors:
1*Neeraj Kumar Lohani, 2Vachaspati Mishra, 3Divakar Joshi
1,2Department of Biotechnology, Institute of Biomedical Education and Research, Mangalayatan University, Beswan, Aligarh-Uttar Pradesh, india,
3MBPG College Haldwani Nainital Kumaun University Nainital Uttarakhand.
neerajlohani06@gmail.com
{ DOWNLOAD AS PDF }
Abstract
The interdisciplinary approach with nanotechnology and animal tissue culture technique is going to revolutionize biomedical science in the next fifty years. Nanotechnology along with regulated animal tissue culture, makes tissue engineering a realization based on the creation of new tissues in vitro followed by surgical placement in the body or the stimulation of normal repair in situ using bio-artificial constructs or implants of living cells introduced in or near the area of damage at nano level.It makes use of artificially stimulated cell proliferation by using suitable nano-material based scaffolds and growth factors. Nanotechnology can be successfully used to create a tissue or organ that can take the place of one that is terminally diseased, such as an eye, ear, heart, or joint. Implantable prosthetic devices and nano scaffolds are used for growing of artificial organs. The key components of tissue engineering with nanotechnology include: cells, scaffolds, signals and bioreactors. Scaffolds are produced by electro-spinning technique.The scaffold acts as an interim synthetic extra cellular matrix (ECM) that cells interact with prior to forming a new tissue.
Nano materials such as quantum dots, fluorescent carbon nano tubes and fluorescent magnetic nano particles, etc., are been used for imaging and tracing and for gene or drug delivery. Designed nanostructures have been used to regulate the proliferation and differentiation of stem cells, which will speed up the understanding and controlling the micro environmental signals, helping to solve the current bottleneck problems of tissue -based therapy. In the future, we could imagine a world where medical nano devices are routinely implanted or even injected into the bloodstream to monitor wellness and to automatically participate in the repair of systems that deviate from established norms.
REFERENCE ID: PHARMATUTOR-ART-2082
INTRODUCTION
The field of tissue engineering has developed in phases: initially researchers searched for “inert” Biomaterials to act solely as replacement structures in the body (Hoerstrup SP et al., 2004).. Then, they explored biodegradable scaffolds both naturally derived and synthetic for the temporary support of growing tissues. Discoveries in nanotechnology have driven both our understanding of cell–substrate interactions, and our ability to influence them. By operating at the size regime of proteins themselves, nanotechnology gives us the opportunity to directly speak the language of cells, through reliable, repeatable creation of nanoscale features. Understanding the synthesis of nanoscale materials, via “top-down” and “bottom-up” strategies, allows researchers to assess the capabilities and limits inherent in both techniques(Wang X et al.,2002).
Novel materials are being developed with unique utility for ultrasensitive detection of biomolecules, for targeted delivery of therapeutic agents directly to affected cells and tissues in the body, and as a tissue scaffold to promote healing. Novel diagnostic methods and treatments are emerging from our increasing ability to control the synthesis of materials such as quantum dots, dendrimers, hydrogels and nanotubes, and to develop methods for optimizing the properties of these materials in living biological systems(Breedveld V. et al.,2004).In parallel, we are learning how to make nano materials that can be used safely and effectively for many other types of consumer products.
Nanotechnology provides the field of medicine with promising hopes for assistance in diagnostic and treatment technologies as well as improving quality of life. Humans have the potential to live healthier lives in the near future due to the innovations of nanotechnology. Some of these innovations include:
· Disease diagnosis
· Prevention and treatment of disease
· Better drug delivery system with minimal side effects
· Tissue Reconstruction
TISSUE RECONSTRUCTION
Nanoparticles can be designed with a structure very similar to the bone structure. An ultrasound is performed on existing bone structures and then bone-like Nanoparticles are created using the results of the ultrasound(Simon Timothy M et al.,1999). The bone-like nanoparticles are inserted into the body in a paste form. When they arrive at the fractured bone, they assemble themselves to form an ordered structure which later becomes part of the bone.
Another key application for nanoparticles is the treatment of injured spinal cord (Bundesen L. Q. et al., 2003). Samuel Stupp and John Kessler at Northwestern University in Chicago have made tiny rod like nano-fibers called amphiphiles .They are capped with amino acids and are known to spur the growth of neurons and prevent scar tissue formation. Experiments have shown that rat and mice with spinal injuries recovered when treated with these nano-fibers.
METHODS FOR NANOFIBER SYNTHESIS
Currently, there are three techniques available for the synthesis of nanofibers: electrospinning, self-assembly, and phase separation. Of these, electrospinning is the most widely studied technique and also seems to exhibit the most promising results for tissue engineering applications (Ahmed, I et al., 2006, frenot A et al., 2003).Nanofibers synthesized by self-assembly and phase separation have had relatively limited studies that explored their application as scaffolds for tissue engineering (Hartgerink JD et al., 2002). Although there are a number of techniques for the synthesis of carbon nanofibers, such as chemical vapor deposition using a template method, catalytic synthesis (catalytic deposition, floating catalyst method, synthesis using radio frequency supported microwave plasmas, the description of each of these techniques is beyond the scope of this review (Hammel E et al., 2004). Therefore, for carbon and alumina nanofibers, the discussion is restricted to their applications in tissue engineering.
Electrospinning Technique
Electrospinning represents an attractive technique for the processing of polymeric biomaterials into nanofibers (Boudriot, U. and R. D. A. G. J. H. Wendorff 2006). This technique also offers the opportunity for control over thickness and composition of the nanofibers along with porosity of the nanofiber meshes using a relatively simple experimental setup.
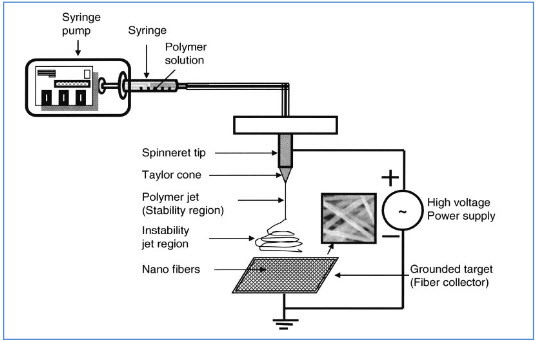
Figure 1: An electrospinning device with a plate-shape metallic collector. The metallic plate can be either stationary or rotating to achieve different orientations of electrospun nanofibers (Reprinted from Tissue Engineering with permission of Mary Ann Liebert)
Although the electrospinning process is relatively simple in terms of its output, an understanding of the underlying mechanisms and several key processing conditions are essential for effective control of fiber properties. As the polymer solution is pumped to the spinneret tip, the high voltage induces charge accumulation to the solution. The droplet is then elongated into a conical shape, known as a Taylor cone due to the electrostatic repulsive force. Electrospinning is initiated when the repulsive force of the solution exceeds the surface tension of the droplet. A finely charged jet is formed at the tip of the Taylor cone. The jet is then stretched and accelerated, accompanied by solvent evaporation, and eventually collected by the target. Adjustment of the applied DC voltage, feeding rate, polymer solution viscosity and working distance can be used to control the morphology of the collected fibers. Here, some key effects of these processing parameters are briefly introduced; In most cases, increasing the applied DC voltage and resultant electric field will cause greater stretching of the polymer solution, which consequently reduces the diameter of the fibers. However, too high a voltage at a given feed rate will lead to a smaller and less stable Taylor cone, which can cause larger diameter bead formation along the fibers. The feed rate will also determine the volume of the polymer solution available for electrospinning at the spinneret tip. At a given voltage, higher feed rates generally yield fibers with larger diameters, although these rates are accompanied by slow solvent evaporation during flight time, leading to residual solvent and fusion of fibers. Working distance has less of an influence on fiber morphology, but smaller working distances results in an increased electric field strength and reduced flight time, which may also cause bead formation and fiber fusion.
The process of electrospinning is affected by two sets of parameters: system parameters and process parameters. System parameters such as polymer molecular weight and distribution determine the rate of degradation of nanofibers. System parameters such as polymer solution properties, i.e., viscosity, surface tension, and conductivity, determine the nanofiber diameter and reduce the possibility of bead formation. Process parameters such as orifice diameter, flow rate of polymer, and electric potential influence fiber diameter. Process parameters such as distance between capillary and metal collector determine the extent of evaporation of solvent from the nanofibers, and deposition on the collector, whereas motion of collector determines the pattern formation during fiber deposition. The systemic and process parameters vary with different polymeric systems and in most cases lend themselves to modification, thereby enabling tailoring of nanofibers for specific end uses (Fridrikh et al., 2003).
Nanofibrous scaffolds & adhesion:
Electrospun poly(L-lactic acid) (PLLA) Nanofibrous scaffolds show significant improvement for neural stem cell adhesion in vitro compared with those cultured on solvent cast flat PLLA surfaces (Baker et al., 2008, Geng X et al.,2005). PC12 cells cultured on either flat or nanostructured surfaces in close proximity migrate from the flat region towards the nanostructured region of the surface .A commonly held explanation for the enhanced adhesion is the increase in surface roughness and surface area, leading to an increase in nonspecific protein adsorption and availability of focal adhesion sites.
Carbon Nanotubes and Tissue Engineering
Carbon nanotubes, because of unique mechanical, physical, and chemical properties, have great potential applications in various fields including molecular electronics, medical chemistry, and biomedical engineering (Y. Liuet al., 2005). Carbon nanotubes can be functionalized to achieve improved properties and functions such as biocompatibility and bio molecular recognition capabilities (Q. Lu et al., 2004). Protein conjugated CNTs can move across the cellular membrane and enter into cytoplasm and cell nucleus ( Funhoff AM et al., 2005, N.W.S. Kam et al., 2004).Patterned growth of mouse stem cells on large scale carbon nanotubes pattern have been successfully undertaken on a non-polar 1- octadecanethiol (ODT) while leaving some bare silver area have been successfully undertaken.
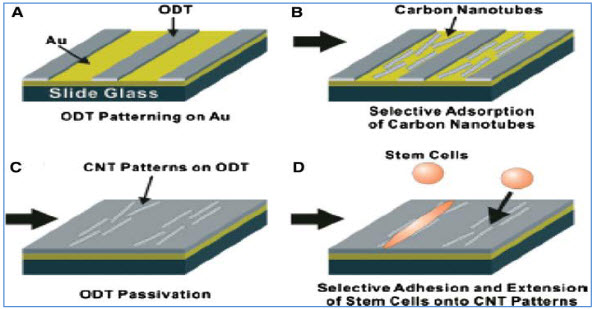
Figure 2: Schematic diagram of carbon nanotubes patterned growth of mouse stem cells.
Nanotechnology and Stem Cells
Nanotechnology and biomedical treatments using stem cells (such as embryonic stem cell) are among the newest veins of biotechnological research(Bain et al., 1995). Stem cells along with nanotechnology have various applications in tissue engineering(Ao, Q. et al., 2007).
Table 1:-Types and sources of human stem cells along with nanotechnology for tissue engineering.
|
Origin |
Types of stem cells |
Source of isolation |
|
Adult |
Umbilical cord matrix stem cells |
Wharton’s jelly |
|
|
Umbilical cord blood stem cells |
Umbilical cord blood |
|
|
Epidermal derived stem cells |
Skin, hair |
|
|
Muscle derived stem cells |
Muscles |
|
|
Adipose derived stem cells |
Adipose tissue |
|
|
Neural stem cells |
Neural tissue |
|
|
Hematopoietic stem cells |
Bone marrow and peripheral blood |
|
|
Mesenchymal stem cells |
Bone marrow |
|
Embryonic |
Embryonic stem cells |
Inner cell mass of 5-7 days of blastocytes |
|
|
Embryonic germ cells |
Gonadal ridge of 6-11 week fetus |
Although both electrospun and self-assembled peptide fibrous scaffolds show promising results in nerve regeneration after PNS/CNS injuries (Beniashet al., 2005). There is still an essential requirement to further investigate the cellular response in vivo with scaffolds (Belle et al., 2004). The importance of transplantation of stem/progenitor cells arises because of the complicated dynamic in vivo microenvironment. The presence of proteins with non-specific adsorption properties on the scaffold surface can possibly cause unexpected outcomes in vivo (Borkenhagen, et al., 1998).
The current “design rules” of tissue engineering scaffolds used for nerve repair only mimic a small part of the structural features of natural ECM and incorporate a limited number of biochemical cues that are known to promote functional recovery of injured nerves. The inflammation and foreign body response due to the introduction of the scaffold and transplanted cells needs to be controlled to reduce further activation of cells like microglia, astrocytes and oligodendrocytes which contribute to the inhibitory environment. Studies using scaffolds with/without transplanted cells for nerve regeneration are largely based on animal models(Carlberg et al., 2009, Blight, A. R. 1994). However, in vivo studies in patients will be essential to evaluate the effectiveness of such neural tissue engineering approaches as therapeutic strategies. Many challenges lay ahead to achieve successful nerve regeneration using scaffolds (Gersbach CA et al., 200).. The current regenerative outcome obtained both in vitro and in vivo in animal models using nanostructured scaffolds, reveals the exciting possibility of manipulating cell behavior that can promote cell survival, neurite outgrowth, appropriate reinnervation and consequently the functional recovery post PNS/CNS injuries (Busch, S. A. and J. Silver 2007).
APPLICATIONS OF NANOSTRUCTURED SCAFFOLDS IN BIOLOGY AND MEDICINE:
Nanofibers in tissue engineering
A variety of methods has been reported previously for the fabrication of scaffolds to be used in tissue engineering (Goulet F. et al., 2000, Stitzel J. et al., 2006). However, in the past decade, nanofibrous systems have been developed and explored as potential scaffolds for tissue engineering. By virtue of their high surface area and porosity, they have the potential to provide enhanced cell adhesion and by virtue of the similarity of their 3D architecture to natural ECM, they provide an excellent micro/nano environment for cells to grow and perform their regular functions. Therefore, Nanofibrous systems have been strongly pursued as scaffolds for tissue engineering applications.
Nanofibers for musculoskeletal tissue engineering
Many materials (natural and synthetic) have been explored as Nanofibrous scaffolding materials for bone, cartilage, ligament, and skeletal muscle tissue engineering, including HA, chitosan, PLGA, carbon and aluminum nanofibers (Hartgerink JD et al., 2001). Although nanofibers have been studied as scaffolds for multiple tissue types, musculoskeletal tissue is probably the most well studied.
Nanofibers for bone tissue engineering
The design of scaffolds for bone tissue engineering is based on the physical properties of bone tissue such as mechanical strength, pore size, porosity, hardness, and overall 3Darchitecture. For bone tissue engineering, scaffolds with a pore size in the range of 100–350 μm and porosity greater than 90% are preferred for better cell/tissue in-growth and hence enhanced bone regeneration. In another study, used HA with β-tricalciumphosphate (β-TCP) to develop biodegradable nanocompositeporous scaffolds (Ramay and Zhang 2004). β-TCP/HA scaffolds built from HA nanofibers with β-TCPas a matrix were used to fabricate porous scaffolds by a technique that integrated the gel casting technique with the polymer sponge method. There in vitro results demonstrated that incorporation of HA nanofibers as a second component in β-TCP significantly increased the mechanical strength of the porous composite scaffolds. This study introduced nano-composites with HA nanofibers as a promising scaffolding system for load bearing applications such as bone tissue engineering (Du C et al.,1998).
Nanofibers for skin tissue engineering
Skin wounds normally heal by formation of epithelialized scar tissue rather than by regeneration of full skin. the two layers of skin, epidermis and dermis, the epidermis has less capacity to heal; however, when large areas of the epidermis need to be replaced, normal regeneration is lacking. Further, the dermis has an enormous capacity to regenerate. The scar tissue that forms in the absence of dermis lacks elasticity, flexibility, and strength of the normal dermis. Consequently, scar tissue limits movements, causes pain, and is cosmetically undesirable. Therefore, engineered skin tissue would be an excellent alternative, not only to close the wound but also to stimulate the regeneration of the dermis. Along with collagen, several other natural and synthetic polymers have been explored for skin tissue engineering however, the use of these biomaterials as nanofibers has been very limited. Developed nonwoven silk fibroin nanofibers by electrospinning for skin tissue engineering. Due to their high porosity and high surface area to volume ratio, fibroin nanofibers coated with type I collagen were found to promote keratin ocytes/fibroblast adhesion and Spreading.
Therefore, the silk fibroin nanofibers show potential to be developed as a scaffold for skin tissue Engineering. Studied PU electrospun nanofiber membranes for the purpose of wound dressings. The PU nanofiber membranes provided excellent oxygen permeability and controlled water evaporation. By virtue of these properties, the nanofiber membranes allowed fluid from the wound to exude out while preventing dehydration of the wound. Further, the ultra-fine porosity of the PUnanofiber membranes disallowed invasion by exogenous microorganisms. These results indicated that the PU nano fiber membranes showed potential to be developed as wound dressing materials.
Nan fibers for neural tissue engineering
In the nervous system, degeneration of neurons or glial cells or any unfavorable change in the extracellular matrix of neural tissue can lead to a wide variety of clinical disorders. Neural tissue repair is a daunting challenge because almost all neural injuries lead to an irreversible loss of function. Neural tissue engineering aims to repair neural tissue by employing biological tools such as normal or genetically engineered cells and ECM equivalents along with potent synthetic tools such as biomaterials for scaffold design and/or drug delivery systems.
Nanofibers for controlled drug delivery
Controlled delivery systems are used to improve the therapeutic efficacy and safety of drugs by delivering them to the site of action at a rate dictated by the need of the physiological environment. A wide variety of polymeric materials have been used as delivery matrices, and the choice of the delivery vehicle polymer is determined by the requirements of the specific application (Heller and Hoffman 2004). Polymeric nanofibers have recently been explored for their ability to encapsulate and deliver bioactive molecules for therapeutic applications.
CONCLUSIONS
Nanotechnology is still in its early stages. The applications discussed in this report have already been developed and are already helping patients all over the world. As further research continues in this field, more treatments will be discovered. Mimicking the architecture of ECM is one of the major challenges of tissue engineering. Amongst all the approaches used to prepare ECM synthetically, the approach using nanofibers has shown the most promising results. Nanofibers can be formed using either one of the three prevailing techniques: electrospinning, self-assembly, or phase separation. Electrospinning is the most widely studied technique and has also shown the most promising results. The availability of a large range of natural and synthetic biomaterials has fueled the area of nanofiber synthesis, especially using the electrospinning technique.
Nano materials such as quantum dots, fluorescent CNTs and fluorescent MNPs, etc., have been used for imaging and tracing, gene or drug delivery, scaffolds for tissue engineering, designed nanostructures have been used to regulate the proliferation and differentiation of stem cells, which will speed up the understanding and controlling the micro environmental signals, helping to solve the current bottleneck problems of tissue -based therapy.
REFERENCES
•Ahmed, I., H.-Y. Liu, et al. "Three-dimensional nanofibrillar surfaces covalently modified with tenascin-C-derived peptides enhance neuronal growth in vitro." Journal of Biomedical Materials Research Part A. 2006, 76A(4): 851-860.
•Ao, Q., A. J. Wang, et al. "Combined transplantation of neural stem cells and factory unsheathing cells for the repair of spinal cord injuries." Medical Hypotheses. 2007, 69(6): 1234-1237.
•Bain, G., D. Kitchens, et al. "Embryonic stem cells express neuronal properties in Vitro." Developmental Biology. 1995, 168(2): 342-357.
•Baker, B. M., A. O. Gee, et al. "The potential to improve cell infiltration in composite fiber-aligned electro spun scaffolds by the selective removal of sacrificial fibers." Biomaterials. 2008, 29(15): 2348-2358.
•Belle, J. E. L., M. A. Caldwell, et al. "Improving the survival of human CNS precursor-derived neurons after transplantation." Journal of Neuroscience Research. 2004, 76(2): 174-183.
•Beniash, E., J. D. Hartgerink, et al. "Self-assembling peptide amphiphiles nanofiber matrices for cell entrapment." Act a Biomaterial. 2005, 1(4): 387-397.
•Blight, A. R. "Effects of silica on the outcome from experimental spinal cord injury: Implication of macrophages in secondary tissue damage." Neuroscience. 1994, 60(1): 263-273.
•Borkenhagen, M., R. C. Stoll, et al. "In vivo performance of a new biodegradable polyester urethane system used as a nerve guidance channel." Biomaterials. 1998, 19(23): 2155-2165.
•Boudriot, U. and R. D. A. G. J. H. Wendorff. "Electro spinning Approaches Toward Scaffold Engineering: A Brief Overview." Artificial Organs. 2006, 30(10): 785-792.
•Breedveld, V., A. P. Nowak, et al. "Rheology of block co polypeptide solutions: hydro gels with tunable properties." Macromolecules. 2004, 37(10): 3943-3953.
•Bundesen, L. Q., T. A. Scheel, et al. "Ephrin-B2 and EphB2 regulation of astrocytemeningeal fibroblast interactions in response to spinal cord lesions in adult rats." Journal of neuroscience. 2003, 23(21): 7789-7800.
•Busch, S. A. and J. Silver. "The role of extracellular matrix in CNS regeneration."Current Opinion in Neurobiology. 2007, 17(1): 120-127.
•Carlberg, B., M. Z. Axell, et al. "Electro spun polyurethane scaffolds for proliferation and neuronal differentiation of human embryonic stem cells." Biomedical Materials. 2009, 4(4): 045004.
•Du C., Cui F.Z., Feng Q.L., Zhu X.D. and de Groot K., “Tissue Response to Nano Hydroxyapatite/Collagen Composite Implants in Marrow Cavity”, J. Biomed. Mater. Res., 42, 540-548, 1998.
•Frenot A, Chronakis I. 2003. Polymer nanofiber assembled by electro spinning. Curr Opin Coll Inter Sci, 8:64–75.
•Fridrikh SV, Yu JH, Brenner MP, et al. Controlling the fiber diameter during electro spinning. Phys Rev Lett, 2003, 90:144502–1, 144502–4.
•Funhoff AM, Monge S, Teeuwen R, et al. PEG shielded polymeric double-layered micelles for gene delivery. J Control Release, 2005, 102:711–24.
•Geng X, Kwon OH, Jang J. Electro spinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials, 2005, 26:5427–32.
•Gersbach CA, Byers BA, Pavlath GK, et al. Runx2/Cbfa1-genetically engineered skeletal myoblasts mineralize collagen scaffolds in vitro.Biotechnol Bioeng, 2004, 88:369–78.
•Goulet F, Rancourt D, Cloutier R, et al. Tendons and ligaments. InLanza RP, Langer R, Vacanti J (eds). Principles of tissue engineering.2nd ed. San Diego: Academic Pr. 711–22., 2000
•Hammel E, Tang X, Trampert M, et al. Carbon nanofiber for composite applications. Carbon, 2004, 42:1153–8.
•Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science, 2001, 294:1684–8.
•Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers:a versatile scaffold for the preparation of self assembling materials.Proc Natl Acad Sci U S A, 2002, 99:5133–8.
•Heller J, Hoffman AS. Drug delivery system. In Ratner BD, Hoffman AS, Schoen FJ, et al (eds). Biomaterial science: an introduction to materials in medicine. 2nd ed. San Diego: Elsevier Academic Pr.p 629–48., 2004
•Hoerstrup SP, Vacanti JP. Overview of tissue engineering. In Ratner BD, Hoffman AS, Schoen FJ (eds). Biomaterial science: an introduction to materials in medicine. 2nd ed. San Diego: Elsevier Academic Pr. p 712–27. 2004
•N.W.S. Kam, T.C. Jessop, P.A. Wender, H. Dai, J. Am. Chem.Soc. 126, 6850. 2004
•Q. Lu, J.M. Moore, G. Huang, A.S. Mount, A.M. Rao, L.L.Larcom, P.C. Ke, Nano Lett. 4, 2473. 2004
•Stitzel J. et al control fabrication of a biological vascular substitution. Biomaterials 27:1088-1094. 2006
•Simon Timothy M et al, Clinical Orthopaedics and Related Research, October 1999- Volume 367, pp S31-S45
•Wang X., Li Y., Wei J. and de Groot K., “Development of Biomimetic Nanohydroxyapatite/Poly (Hexamethylene Adipamide) Composites”, Biomaterials, 23, 4787-4791, 2002.
•Y. Liu, D. Wu, W. Zhang, X. Jiang, C. He, T.S. Chung, S.H. Goh, K.W. Leong, Angew. Chem. Int. Ed. 44, 4782, 2005.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 1 Received On: 09/012/2014; Accepted On: 15/12/2014; Published On: 15/01/2014 How to cite this article: NK Lohani, V Mishra, D Joshi, A New Promise: Neural Tissue Engineering using Nanotechnology, PharmaTutor, 2014, 2(1), 13-20 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









