 About Authors:
About Authors:
Patel Chirag J*, Asija Rajesh, Asija Sangeeta
Maharishi Arvind Institute of Pharmacy, Department of Pharmaceutics, Jaipur.
*chirag.bangalore@gmail.com
ABSTRACT
Oral delivery of drugs with poor aqueous solubility and poor enzymatic and/or metabolic stability is very challenging. However, the advent of nanotechnology has revolutionized the field of oral drug delivery. Development of poorly soluble and/or permeable drug molecules using nanocrystal formulations has proven to be highly successful due to the greater surface/volume ratio, resulting in improvements in dissolution and bioavailability as well as enhanced permeability.The industrially relevant bottom up (precipitation) and top down production technologies (pearl milling, high pressure homogenization, and combination technologies) are presented. This review discusses drug loading among various nanoparticles, method of preparation, evaluation and success of nanocrystals compared to other nanotechnologies.
REFERENCE ID: PHARMATUTOR-ART-1473
INTRODUCTION
Nanocrystals are nanoscopic crystals of the parent compound with dimensions less than 1 µm1. Over the past two decades, there has been continued interest and success in utilizing nanoparticles in the development of pharmaceuticals. Regulatory approval and cost of nanoparticles have been the main limiting factors, with less than 30 marketed formulations since Ambisome (liposomal amphotericin B) was first approved in 19902, 3.
Oral route is the most preferred route for administration of drugs as it offers greatest degree of patient compliance. Poor aqueous solubility and intrinsic dissolution rate (mass of the drug dissolved per time unit and area) are the major factors that affect oral delivery of many existing drugs. Moreover, around 40% of the new chemical entities generated via drug discovery screens exhibit poor aqueous solubility4. Typically, such drugs belong to Class II or IV as per Biopharmaceutical Classification System (BCS) and their oral delivery often results in low bioavailability, erratic absorption, large variations in intra- and inter-subject pharmacokinetics and lack of dose proportionality. Drugs with acceptable solubility but poor membrane permeability are classified as BCS Class III drugs. Usually, these drugs have to be administered at a high dose to achieve therapeutic concentration1.
Formation of crystalline nanoparticles or nanocrystals can improve both solubility and permeability and ultimately bioavailability. According to the Noyes–Whitney equation, a decrease in particle size will lead to an increase in effective surface area in the diffusion layer, which, in turn, increases the drug dissolution rate. Increase in surface area to volume ratio will result in increased cellular uptake due to the 100 fold size reduction5. Animal studies have shown that oral bioavailability is greater for solid lipid nanoparticles compared to nanocrystals, the nanocrystal formulation presents a significant improvement which is both simpler and scalable using various existing technologies6, 7.
ADVANTAGES1
1. Fast dissolution rates
2. Improved dose-bioavailability
3. Established manufacturing techniques
4. Good compatibility with drugs having different solubility profiles
5. Proportionality and increased patient compliance via reduction of number of oral units to be taken
6. Excellent for oral formulations
DISADVANTAGES1
1. Require stabilizers
2. Not ideal for intravenous administration
3. High-energy input
4. Not suitable for cytotoxic drugs with small therapeutic indices
5. Lack of controlled release
HIGH DRUG LOADING AMONG VARIOUS NANOPARTICLES
Lipid nanocarriers such as polymeric nanoparticles, solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC) and nanocrystal have attracted great attention in oral drug delivery. SLN are composed of biodegradable lipids like highly purified triglycerides, monoglycerides, hard fats, complex glyceride mixtures or even waxes which are solid at physiological temperature whereas NLC contain a mixture of blend of a solid and a liquid lipid (oil) in appropriate proportions. The presence of liquid lipid in the NLC results in long-term colloidal stability and higher drug encapsulation and loading unlike SLN8, 9. However, because of the presence of solid lipids, SLN and NLC have the ability to sustain the therapeutic levels in drug in the plasma unlike SES10.The matrix particles have drug distributed throughout the matrix and/or adsorbed onto their surface (drug loading <<100%); the nanocrystals consist of nearly 100% drug11.
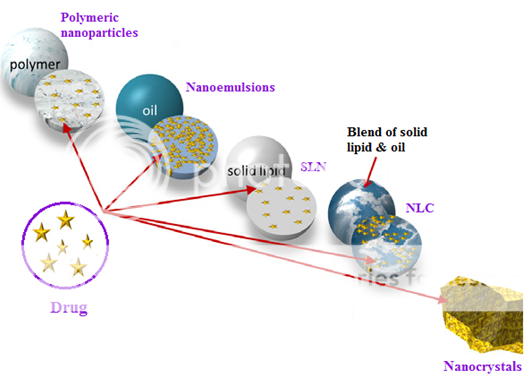
Figure 1: Basic structure of polymeric nanoparticles, nanoemulsions, solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) (means all matrix particles) and nanocrystals.
PREPARATION OF NANOCRYSTALS1, 11
Two basic approaches are involved in production of nanocrystals, the bottom-up technologies (controlled precipitation/crystallization) and the top-down technologies, nanonizing (large-size drug powder to be reduced in size, e.g. by mechanical attrition). However, the combination techniques, combining a pre-treatment with a subsequent size reduction step are also being employed.
1. Bottom-up-precipitation methods
Bottom–up-precipitationtechnology is basically a classical precipitation process known as “via humida paratum” (VHP), where drug is dissolved in a solvent and subsequently precipitated by mixing with a non-solvent. It yields crystalline drug nanoparticles. This method requires strict control of the process, avoidance of crystal growth to the micrometer range, drug solubility in at least one solvent, and of course has the problem of solvent residues. Due to the complexity of process, as per our knowledge there are no pharmaceutical products on the market based on this technology.
Another precipitation process was developed by Auweter and Horn, leading to amorphous nanoparticles. The particles are spherical due to precipitation process. This process is used by BASF for products developed in the food sector (e.g. Lucarotin® or Lucantin® which is a solution of the carotenoid, together with a surfactant in a digestible oil), and for pharmaceuticals by Soliqs® previously Knoll/BASF. The Soliqs trade name is NanoMorph®. Theoretically, a particle being in the nano range and at the same time amorphous is ideal; it has the highest increase in saturation solubility. However, there is a risk that the amorphous active can re-crystallize; in this case pharmaceutical product leads to a decrease in the oral bioavailability. Partial re-crystallization is less or not critical in food products, therefore by now products in this sector are on the market. After introduction of crystalline nanoparticles to the market, amorphous nanoparticles might belong to the second improved generation, because of their superior dissolution velocity and higher solubility.
Another bottom–up process is controlled crystallization during freeze drying, which is also considered to be suitable for large-scale production
2. Top–down technologies
2.1 Bead/pearl milling
The NanoCrystals® technology by élan uses a bead/pearl mill to achieve particle size diminution. Milling media, dispersion medium (generally water) containing stabilizer along with drug are charged into a milling chamber. Shear forces generated by the movement of the milling media lead to particle size reduction. Smaller or larger coated milling pearls of ceramics, stainless steel, glass or highly cross linked polystyrene resin-coated beads can be used. Erosion from the milling material during the milling process is a common problem of this technology. The milling time mainly depends on the hardness of the drug, viscosity, temperature, energy input, size of the milling media and surfactant concentration used. The milling time can last from about 30 min to hours or several days. This is an important industrially used technology for particle size reduction, proven by the FDA-approved products. Typically lab scale production can be carried out at using 100mg or less of API by using the Nanomill® system. The chemical form of API needs to be considered for laboratory testing, typically the neutral form is preferred. Production volumes of more than 5 L (flow through mode) can be produced using the Dynomill (Glen Mills, Inc. Cliffton, NJ, USA) with chamber size of 300 and 600 mL. Also larger sized mills are available (e.g. Netzsch mills), e.g. in 2, 10 and 60 L chamber size. Scaling up with a pearl mill is possible, but there is a certain limitation in size of the mill due to its weight. To produce larger batches the mills can be configured in the circulation mode. The NanoCrystal® technology has successfully expanded the use of nanosuspensions for oral, inhalation, intravenous, subcutaneous, intramuscular and ocular delivery.
2.2 High pressure homogenization
The three basic processes used are Microfluidizer technology (IDD-PTM technology) based on the jet stream principle, piston-gap homogenization either in water (Dissocubes® technology, SkyePharma) or alternatively in water-reduced/non-aqueous media (Nanopure® technology, prev. PharmaSol GmbH, Berlin, now Abbott Laboratories). The Microfluidizer technology is based on the jet stream principle and can generate small particles by a frontal collision of two fluid streams in a Y-type or Z-type chamber under pressures up to 1700 bar. The jet streams lead to particle collision, shear forces and cavitation forces (Microfluidizer®, Microfluidics Inc.). Often a relatively high number of cycles (50–100 passes) are necessary to obtain sufficient particle size reduction. SkyePharma Canada Inc. uses this principle for their Insoluble Drug Delivery-Particles (IDD-PTM) technology to achieve the production of submicron particles of poorly soluble drugs. Product on the market is Triglide®, fenofibrate. The Dissocubes® technology was developed by Müller and co-workers by employing piston-gap homogenizers (e.g. APV Gaulin/Rannie homogenizers). The technology was acquired by SkyePharma PLC in 1999. A drug dispersed in an aqueous surfactant solution (macrosuspension) is forced by a piston under pressure (up to 4000 bar, typically 1500–2000 bar) through a tiny gap. The resulting high streaming velocity of the suspension causes an increase in the dynamic pressure. This is compensated by a reduction in the static pressure below the vapor pressure of the aqueous phase; hence, water starts boiling forming gas bubbles. These gas bubbles collapse immediately when the liquid leaves the homogenization gap. The drug particles are reduced in size due the high power of the shockwaves caused by cavitation. The mean size of bulk population obtained for the high pressure homogenization process depends on the power density of the homogenizer (homogenizer pressure), number of homogenization cycles and hardness of drug. Because of crystalline nature they appeared in cuboid or irregular shape, in contrast to spherical amorphous drug nanoparticles. The Nanopure® technology is another approach using the piston-gap homogenizer. This technology uses a primary dispersion medium, non-aqueous liquids, e.g. oils, liquid and solid (melted) PEG, or water reduced media (e.g. glycerol–water, ethanol–water mixtures), and optionally homogenization at low temperatures. These media have low vapor pressure, cavitation takes place very limited or not at all. At homogenization at room temperature, the water starts boiling, i.e. the static pressure on the water is reduced to the vapor pressure of water at 20 ?C, being 23.4. For example, the vapor pressure of Miglyol 812 oil is only 0.01 hPa at 20 ? C, i.e. more than 2000 fold lower. Therefore when water shows cavitation, the oil will not. Even without cavitation, the size diminution is sufficient because of shear forces, particle collisions and turbulences. The optional low temperatures allow the processing of temperature sensitive drugs, in addition at lower temperatures materials, are more fragile. Final nanosuspensions product in oil or PEG can be directly filled into gelatin or HPMC capsules.
2.3 Combination technologies
These technologies combine a pre-treatment step with a subsequent high energy step, for example – but not necessarily – high pressure homogenization. The NANOEDGETM technology by Baxter uses a first classical precipitation step with a subsequent annealing step by applying high energy, e.g. high pressure homogenization. According to the patent claims, the annealing step prevents the growth of the precipitated nanocrystals. Annealing is defined in this invention as the process of converting matter that is thermodynamically unstable into a more stable form by single or repeated application of energy (direct heat or mechanical stress), followed by thermal relaxation. This lowering of energy may be achieved by conversion of the solid form from a less ordered to a more ordered lattice structure. Alternatively, this stabilization may occur by a reordering of the surfactant molecules at the solid–liquid interface.
A problem is the use of organic solvents in the precipitation step. In case of large-scale production relatively large amounts of solvent need to be removed, and removal needs to take place in a sterile production process-making it even more tricky and expensive. The Baxter developments focus mainly on i.v. injectables. Due to potential stability impairment of aqueous nanosuspensions by terminal sterilization (autoclaving, irradiation), in general production has to take place under aseptic conditions, including the homogenization line. The smartCrystal® technology is owned by Abbott and marketed by its drug delivery company Soliqs in Ludwigshafen/Germany. It is a family of various combination processes, a kind of tool box to tailor-make the nanocrystals for each specific application, and It was acquired from PharmaSol GmbH in 2007, and these crystalline nanoparticles complement to the amorphous nanoparticles. Special feature of the processes H69 and H96 is the ability to produce crystals below 100 nm, a range practically not accessible by high pressure homogenization alone. Spray-drying or lyophilization of the drug solution leads to a powder more susceptible to be broken in the subsequent high pressure homogenization step. The smartCrystal technology is considered as the second generation of drug nanocrystals. Injecting nanosuspensions with very small nanocrystals can permit fast dissolution and mimic injection of a solution.
NANOCRYSTALS FORMULATIONS
In the beginning, nanocrystals were considered for use in oral, i.v.12 and pulmonary13 administration routes. After almost twenty years of nanocrystal development, several oral products, one injectable product and no i.v. injectable or pulmonary products are on the market. There are two reasons why we mainly have oral nanocrystal products: the oral market is the largest market segment and oral products are easier to manufacture. Intravenous products are more complex. One idea was to replace the toxicologically problematic i.v. products on the market with well-tolerated injectable nanosuspensions. Examples are Taxol (paclitaxel) containing Cremophor EL (for anaphylactic reactions) and Sporanox (itraconazole) solubilized in cyclodextrin (for nephrotoxicity). However, the injected nanocrystals dissolved too slowly, and the crystals were phagocytosed by liver and spleen macrophages. This resulted in different pharmacokinetics compared to the commercial products, which are solutions14. Generic products could not be produced. Therefore, research on the use of small crystals that dissolve quickly and mimic solution pharmacokinetics is necessary15. Pulmonary products are essentially feasible. Nanosuspensions can be aerosolized using commercial nebulizers16, but no products have been created. The reason may be commercial and not technical. It makes little sense to replace a well-selling product with a nanosuspension simply because pulmonary deposition might be superior. The cost of market introduction is too high. Even with a new molecule, an established routine delivery technology is preferable.
EVALUATION17, 18, 19
1. Particle size
Particle size and size distribution are the most important characteristics of nanoparticulate carrier systems. They determine the in vivodistribution, biological fate, toxicity and the targeting ability of nanoparticulate carrier systems. In addition, particle size and size distribution can also influence the drug loading, drug release and stability of nanocrystals. Currently, photon-correlation spectroscopy or dynamic light scattering is the fastest and also frequently employed method for determining particle size and size distribution. Photon-correlation spectroscopy requires the viscosity of the medium and determines the diameter of the particle by Brownian motion and light scattering properties. The results obtained by photon-correlation spectroscopy are usually verified by scanning or transmission electron microscopy (SEM or TEM). The electron microscopic methods however require coating of Gold (as conducting material). As a result of this coating, particle size observed is larger than normal. Scanning Electron Microscopy is also limited to dry sample. For those nanocrystals which have little electron density are difficult to stain. Transmission Electron Microscopy is a better alternative in such cases. TEM observes the nanocrystals by freeze fracturing or by freeze substitution. This method is also advantageous as it allows observation of the interior of the nanocrystals due to fracturing of these particles. Nanocrystals surface morphology can also be studied by recently developed microscopic techniques like atomic force microscopy and scanning tunneling microscopy.
2. Surface properties of nanoparticles
The zeta potential of nanocrystals is commonly used to characterize the surface charge property of nanocrystals. The electrical potential of particles is reflected by zeta potential and is influenced by the composition of the particles and the medium in which it is dispersed. Nanocrystals with a zeta potential above (+/-) 30 mV have been shown to be stable in suspension, as the surface charge prevents aggregation of the particles.
3. Molecular weight:
Molecular weight of polymers used in formulation of nanocrystals can be determined once dissolution of particles in an appropriate solvent is achieved. The solution is then analyzed by gel permeation chromatography. Accurate results can be obtained only if polymer standards have a similar structure and similar properties as test materials.
4. Density:
Density of nanocrystals can be measured by two methods: by helium compression pycnometry and by density gradient centrifugation. A comparison of these two methods may offer information about internal structure of nanocrystals.
5. Hydrophobicity:
Hydrophobicity can be determined by two methods: by contact angle measurements and by hydrophobic interaction chromatography. The contact angle measurements can be performed on flat surfaces and not directly on hydrated nanocrystals in their dispersion media. For this region hydrophobic interaction chromatography is better method, wherein differentiations between nanocrystals with different surface properties can be obtained by loading particle on column with alkyl-sepharose and eluting them with a triton X-100 gradient.
6. Drug loading
For a nanoparticulate system to be successful, it should have a high drug-loading capacity thereby reducing the quantity of matrix materials for administration. Drug loading can be done by:
i. Incorporating at the time of nanocrystals production (incorporation method)
ii. Absorbing the drug after formation of nanocrystals by incubating the carrier with a concentrated drug solution (adsorption/absorption technique).
Drug loading and entrapment efficiency very much depend on the solid-state drug solubility in matrix material or polymer (solid dissolution or dispersion), which in turn depends on the polymer composition, the molecular weight, the drug polymer interaction and the presence of end functional groups (ester or carboxyl).
7. Drug release
The following methods are used to study in vitro release of drug from nanocrystals:
i. Side-by-side diffusion cells with artificial or biological membranes.
ii. Dialysis bag diffusion technique.
iii. Reverse dialysis bag technique.
iv. Agitation followed by ultracentrifugation.
v. Ultra-filtration or centrifugal ultra-filtration techniques.
The in vitro release study is carried out by controlled agitation followed by centrifugation. Dialysis is preferred over other methods of separation of nanocrystals from the release media as it is less time consuming and pose less technical difficulties.
8. X-ray characterization ofnanocrystals
X-ray characterization is a powerful approach to the study of nanoparticulate systems. The advantage of these techniques is to provide information about both medium range and local atomic structure in nanoparticulate systems.
9. Percentage yield
Percentage yield is an important evaluation parameter when scale up is considered. It is not always possible that a scalable process has maximum % yield amongst the processes used for production and vice versa.
% Yield can be calculated by using following equitation:
% Yield = Actual weight of the product
----------------------------------------- x 100
Total weight of excipients and drug
10. Drug entrapment efficiency
Drug entrapment efficiencycan be calculated by using following equitation:
% Drug entrapment = Mass of drug in nanoparticles
------------------------------------- x 100
Mass of drug used in formulation
SUCCESS OF NANOCRYSTALS COMPARED TO OTHER NANOTECHNOLOGIES20
Many nanotechnology delivery systems have been developed since 1986, the year that liposomes were introduced into the market. (The first cosmetic product was Capture by Dior, and the first pharmaceutical products were introduced around 1990) Most nanosystems remained in the laboratory and very few entered the market. By considering cyclodextrin complexes, micelles and drug-loaded i.v. nanoemulsions as nanosystems, until the introduction of the nanocrystals, liposomes may still have performed the most optimally. Strictly speaking, one should also consider their cosmetic use and commercial success. Liposome cosmetics continue to be sold.
Their performance criteria are the number of products and of course, the related sales. Some pharmaceutical liposome products perform very well, but the costs for treatment and their broad use are too high. An example is Ambisome®: it does not exhibit nephrotoxicity, but the treatment costs are in the range of about USD 1000 per day. However, the treatment costs are approximately USD 10 when using the amphotericin solution. In contrast, nanocrystal technology may be less expensive and have fewer physical stability problems compared to liposomes. With Tricor®, the first nanocrystal blockbuster on the market, annual US sales exceed USD 1 billion. When considering products in clinical trials, judging nanocrystals as the most successful pharmaceutical nanotechnology product of the last twenty years appears justified.
CONCLUSION
Nanotechnology has a great potential in improving oral bioavailability and therapeutic efficacy of an array of drugs. Nanocrystals is fast growing alternative formulation routes being readily used by industry to deal with of poorly soluble and/or permeable drug. Besides the liposomes, the nanocrystals are the most successful nanocarrier when considering the short time between invention and first marketed products. In terms of interaction with cells, they belong to the low risk class of nanoparticles, and they are biodegradable. Further research is required to optimize production of nanocrystals and fully utilize their novel surface properties.
REFERENCES
1. Chen H., Khemtong C., Yang X., Chang X. and Gao J., “Nanonization strategy for poorly water soluble drugs”, Drug Discovery Today, 2011; Vol. 16: 354-360.
2. Dusastre V., Nanomedicine: a matter of rhetoric, Nat Mater, 2006; Vol. 5: 243.
3. Allen T.M. and Cullis P.R., “Drug delivery systems: entering the mainstream”, Science, 2004; 303: 1818-1822.
4. Gursoy R.N. and Benita S., “Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs”, Biomed. Pharmacother., 2004; Vol. 58: 173-182
5. Noyes A.A. and Whitney W.R., “The rate of solution of solid substances in their own solutions”, J. Am. Chem. Soc., 1897; Vol. 19: 930-934
6. Müller R.H., Runge S., Ravelli V., Mehnert W., Thunemann A.F. and Souto E.B., “Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLNW) versus drug nanocrystals”, Int J Pharm, 2006; Vol. 317: 82-89.
7. Müller R.H. and Keck C.M., “Challenges and solutions for the delivery of biotech drugs - a review of drug nanocrystal technology and lipid nanoparticles”, J Biotechnol, 2004; Vol. 113: 151-170.
8. Uner M. and Yener G., “Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives”, Int. J. Nanomed, 2007; Vol. 2: 289–300.
9. Almeida A. and Souto E., “Solid lipid nanoparticles as a drug delivery system for peptides and proteins”, Adv. Drug Deliv. Rev., 2007; Vol. 59: 478–490.
10. Müller R.H., “Cyclosporine-loaded solid lipid nanoparticles (SLN): drug–lipid physicochemical interactions and characterization of drug incorporation. Eur. J. Pharm. Biopharm., 2008; Vol. 68, 535-544.
11. Müller R.H., Gohla S. and Keck C.M. “State of the art of nanocrystals – Special features, production, nanotoxicology aspects and intracellular delivery”, European Journal of Pharmaceutics and Biopharmaceutics, 2011; Vol. 78: 1-9.
12. Merisko-Liversidge E., Sarpotdar P., Bruno J., Hajj S., Wei L., Peltier N., Rake J., Shaw J.M., Pugh S., Polin L., Jones J., Corbett T., Cooper E. and Liversidge G.G., “Formulation and antitumor activity evaluation of nanocrystalline suspensions of poorly soluble anticancer drugs”, Pharm. Res., 1996; Vol. 13: 272–278.
13. Jacobs C. and Müller R.H., “Production and characterization of a budesonide nanosuspension for pulmonary administration”, Pharm. Res., 2002; Vol. 19: 189-194.
14. Rabinow B., Kipp J., Papadopoulos P., Wong J., Glosson J., Gass J., Sun C.S., Wielgos T., White R., Cook C., Barker K. and Wood K., “Itraconazole IV nanosuspension enhances efficacy through altered pharmacokinetics in the rat”, Int. J. Pharm., 2007; Vol. 339: 251-260.
15. Gao L., Zhang D., Chen M., Zheng T. and Wang S., “Preparation and characterization of an oridonin nanosuspension for solubility and dissolution velocity enhancement”, Drug Dev. Ind. Pharm., 2007; Vol. 33: 1332-1339.
16. Hernandez-Trejo N., Kayser O., Steckel H. and Müller R.H., “Characterization of nebulized buparvaquone nanosuspensions – effect of nebulization technology”, J. Drug Target., Vol. 13: 499-507.
17. Bibby D.C., Talmadge J.E., Dalal M.K., Kurz S.G., Chytil K.M., Barry S.E., Shand D.G. and Steiert M., “Pharmacokinetics and biodistribution of RGD-targeted doxorubicinloaded nanoparticles in tumor-bearing mice”, Int. J. Pharm., 2005; Vol. 293: 281-290.
18. Fresta M., Puglisi G., Giammona G., Cavallaro G., Micali N. and Furneri P.M., “Pefloxacin mesilate- and ofloxacinloaded polyethylcyanoacrylate nanoparticles; characterization of the colloidal drug carrier formulation” J. Pharm. Sci., 1995; Vol. 84: 895-902.
19. Govender T., Stolnik S., Garnett M.C., Illum L. and Davis S.S., “PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug”, J. Control. Rel., 1999; Vol. 57: 171-185.
20. Müller R.H. and Keck C.M., “Twenty years of drug nanocrystals: Where are we, and where do we go?” European Journal of Pharmaceutics and Biopharmaceutics, 2012; Vol. 80: 1-3.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









