ABOUT AUTHORS:
1S. Amareshwari*, 2Dr. Nanda Kishore Agarwal
1M.Pharm, Department of pharmaceutical analysis, Nimra College Of Pharmacy
2Professor and head of the department of pharmaceutical chemistry, Nimra College Of Pharmacy,
Jupudi, Ibrahimpatnam, Vijayawada
*amareswarisa@gmail.com
ABSTRACT
The present investigation describes about a simple rapid, accurate, precise and reproducible validated reverse phase HPLC method was developed for the determination of Ceftazidime pentahydrate and Tazobactam sodium in bulk and pharmaceutical dosage forms. The quantification was carried out using Hypersil BDS C18 (150 X 4.6mm, 5 µm) column run in isocratic way using mobile phase comprising of phosphate buffer pH 3.0, acetonitrile, and tetrahydrofuran in the ratio of 60:30:10 with a detection wavelength of 205nm and injection volume of 20µL, with a flow rate of 1.0ml/min. The retention times of the drugs were found to be 3.490min and 2.353min. The linearity ranges of the proposed method lies between 60-140mcg/mL and 7.5-17.5mcg/mL for Ceftazidime pentahydrate and Tazobactam sodium with correlation coefficient of r2=0.999 for both. The assay of the proposed method was found to be 99.38% and 99.26%. The recovery studies were also carried out and mean % Recovery was and found to be 99.91% and 100.84%. The % RSD from reproducibility was found to be <2%. The proposed method was statistically evaluated and can be applied for routine quality control analysis of Ceftazidime pentahydrate and Tazobactam sodium in bulk and in Pharmaceutical dosage form.
REFERENCE ID: PHARMATUTOR-ART-1997
INTRODUCTION
Ceftazidime Pentahydrate is (Z)-(7R)-7-[2-(2-Aminothiazol-4-yl)-2-(1-carboxy-1 methylethoxyimino) acetamido]-3-(1-pyridiniomethyl)-3-cephem-4-carboxylate pentahydrate.
The molecular weight is 636.7, molecular formula is C22H22N6O7S2·5H2O. It is a third generation Cephalosporin with enhanced antibacterial activity against gram negative organisms. Ceftazidime is bactericidal in action, exerting its effect on target cell wall proteins and causing inhibition of cell wall synthesis. It is official in IP and BP. It is used in the treatment of biliary tract infections, lower respiratory tract infections, bone and joint infections and urinary tract infections.
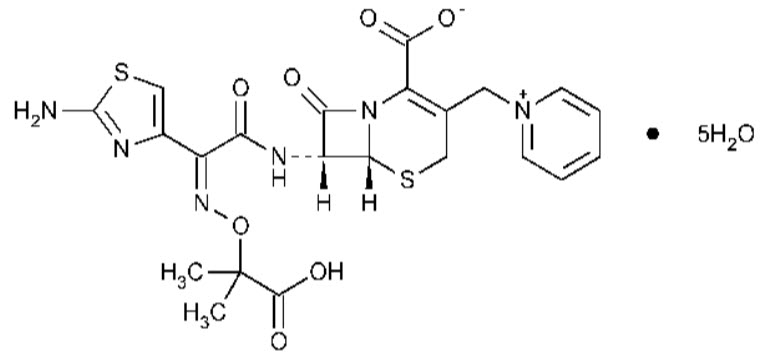
Tazobactam is a potent and novel β-lactamases inhibitor which belongs to the class of penicillanic acid sulfones. The molecular formula is C10H11N4NaO5S, molecular weight is 322.3 and the chemical name is (2S,3S,5R)-3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylic acid 4,4-dioxide. Tazobactam inhibits the action of bacterial beta lactamases. It broadens the spectrum of penicillin by making it effective against organisms that express beta-lactamases degrade piperacillin. Used to reduce the development of drug resistant bacteria.
Literature review reveals that several methods are reported for these drugs alone or in combination with other drugs. For combination of these drugs Spectroscopic method, HPTLC method are reported, there is no single work done for this combination by using RP-HPLC. Hence an attempt has been made for the development of RP-HPLC method for the combination of drugs.
In the present proposed work a successful attempt had been made to develop a method for the simultaneous estimation of Atorvastatin and Fenofibrate pharmaceutical dosage form and validate it. From the economical point of view and for the purpose of routine analysis, the method would help in estimate of drugs in single run which reduces the time of analysis and does not require separate method for each drug. Thus, the paper reports an economical, simple and accurate RP-HPLC method for the above said pharmaceutical dosage forms.
MATERIALS AND METHODS UV-3000 LABINDIA double beam with UV win 5software UV-VISIBLE spectrophotometer with 1cm matched quartz cells. Schimadzu HPLC equipped with SPD 20A UV-VIS detector and the column used was HYPERSIL BDS C18 (150*4.6mm, 5µ). The data acquisition was performed by using LC solutions software. In addition an analytical balance (DENVER 0.1mg sensitivity), digital pH meter (Eutech pH 510), a sonicator (Unichrome associates UCA 701) were used in this study.
CHEMICALS AND REAGENTS
Ceftazidime and Tazobactam pure sample was taken as a gift sample from local labs and dosage form “Combitaz” marketed by LUPIN LABS was purchased from local pharmacy. Other chemicals all are of HPLC grade.
METHODS
Preparation of mobile phase:
A suitable quantity of degassed mixture of pH 3.0 phosphate buffer, Acetonitrile, Tetra hydro furan in the ratio of 60:30:10 was prepared and filtered through 0.45µ filter under vacuum filtration.
Preparation of standard stock solution:
About 100mg of Ceftazidime and 25mg of Tazobactam sodium were weighed and taken into 100mL, 25mL volumetric flasks. To each flask 20mL of diluents were added and sonicated for 15min to dissolve the drugs then made up to required volume with the diluents, to get a concentration of 1mg/ml solutions. From this solution 10mL was taken into a 100mL flask and made up to final volume with diluents to get a concentration of 100ppm filtered through 0.45µ filter under vacuum filtration. From this stock solution further dilutions were made by taking the two drugs in the ratio of 8:1 for the validation of the method developed.
Preparation of the sample solution:
The powder equivalent to 112.53mg of Ceftazidime and Tazobactam sodium were weighed and taken into a 100mL volumetric flask. To this 25mL of diluents was added and sonicated for 15min to dissolve the drugs then made up the volume to required volume with the diluents. From this solution 10ml was taken into a 100mL flask and made up to final volume with diluents to get a concentration of 100ppm filtered through 0.45µ filter under vacuum filtration. From this stock solution further dilutions were made for the validation of the method developed.
METHOD DEVELOPMENT
Method optimization:
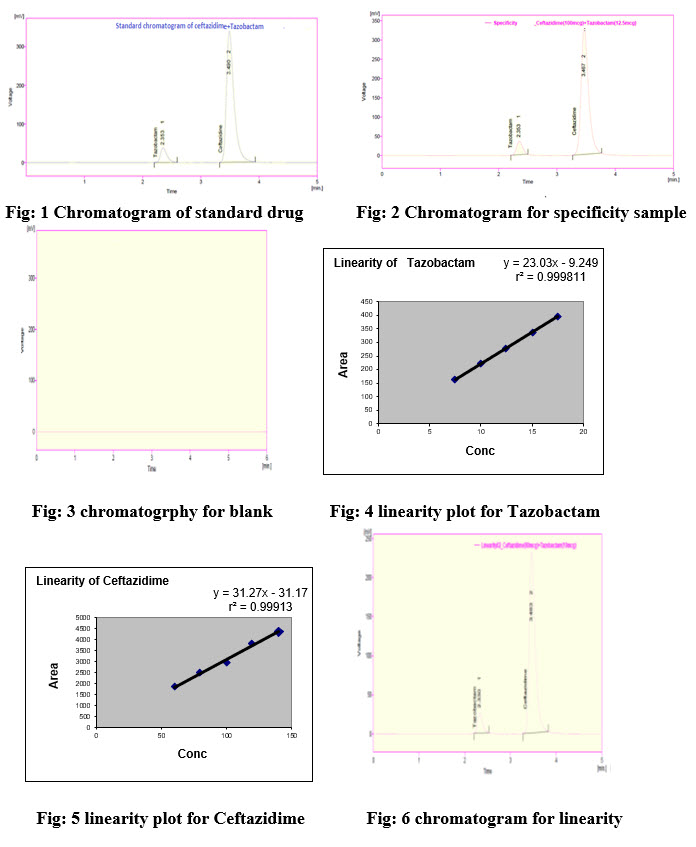
The chromatographic separation was performed usingHypersil BDS C18 (150×4.6mm, 5µm)column. For selection of mobile phase, various mobile phase compositions were observedfor efficient elution and good resolution. The mobile phase consisting of pH 3.0 phosphate buffer, acetonitrile and Tetra hydro furan in the ratio of 60:30:10 was found to be the optimum composition for efficient elution of analyte. The mobile phase was injected to the column at a flow rate of 1.0 ml/min for 8min. The column temperature was maintained at 35 ± 10C. The analyte was monitored at 205nmusing UV-detector. The retention time of the drugs was found to be 3.490 and 2.353min. Mobile phase was used as diluent during the standard and test samples preparation.The optimized chromatographic conditions are mentioned in Table-1 and chromatogram for standard was shown in the figure no: 1.
RESULTS
Method Validation:
1. SPECIFICITY:
Specificity is the ability of analytical method to measure accurately and specifically the analyte in the presence of components that may be expected to be present in the sample. The specificity of method was determined by spiking possible impurities at specific level to standard drug solution (100ppm). The diluent and placebo solutions were also injected to observe any interference with the drug peak. The results are tabulated in the table no-2 and the chromatogram was shown in the figure no- 2, 3.
2. LINEARITY:
Linearity is the ability of the method to produce results that is directly proportional to the concentration of the analyte in samples with given range. The linearity of Ceftazidime was in the concentration range of 60-140%, for Tazobactam sodium 7.5-17.5%. From the linearity studies calibration curve was plotted and concentrations were subjected to least square regression analysis to calculate regression equation. The regression coefficient was found to be 0.999 and shows good linearity for both the drugs. The results are tabulated in the table no-3 and the chromatogram was shown in the figure no-.4, 5, 6.
3. PRECISION:
Precision is the degree of closeness of agreement among individual test results when the method is applied to multiple sampling of a homogeneous sample. Study was carried out by injecting six replicates of the same sample preparations at a concentration of 100ppm/ml. The results are tabulated in the table no-4 and the chromatogram was shown in the figure no-12.
4. ACCURACY:
Accuracy is the closeness of results obtained by a method to the true value. It is the measure of exactness of the method. Accuracy of the method was evaluated by standard addition method. Recovery of the method was determined by spiking an amount of the pure drug (80%,100% ,120%) at three different concentration levels in its solution has been added to the pre analyzed working standard solution of the drug. The results are tabulated in the table no: 4.
5. LOD & LOQ:
LOD is the lowest concentration of analyte in a sample that can be detected but not quantified under experimental conditions. The LOD values were determined by the formulae LOD=3.3σ/s (where σ is the standard deviation of the responses and s is the mean of the slopes of the calibration curves).
LOQ is the lowest concentration of analyte in a sample that can be determined with acceptable precision and accuracy under experimental conditions. It is a parameter of the quantitative determination of compounds in the mixtures. The LOQ values were determined by the formulae LOD=10σ/s. The results are tabulated in the table no: 4
FORCED DEGRADATION OF CEFTAZIDIME AND TAZOBACTAM SODIUM
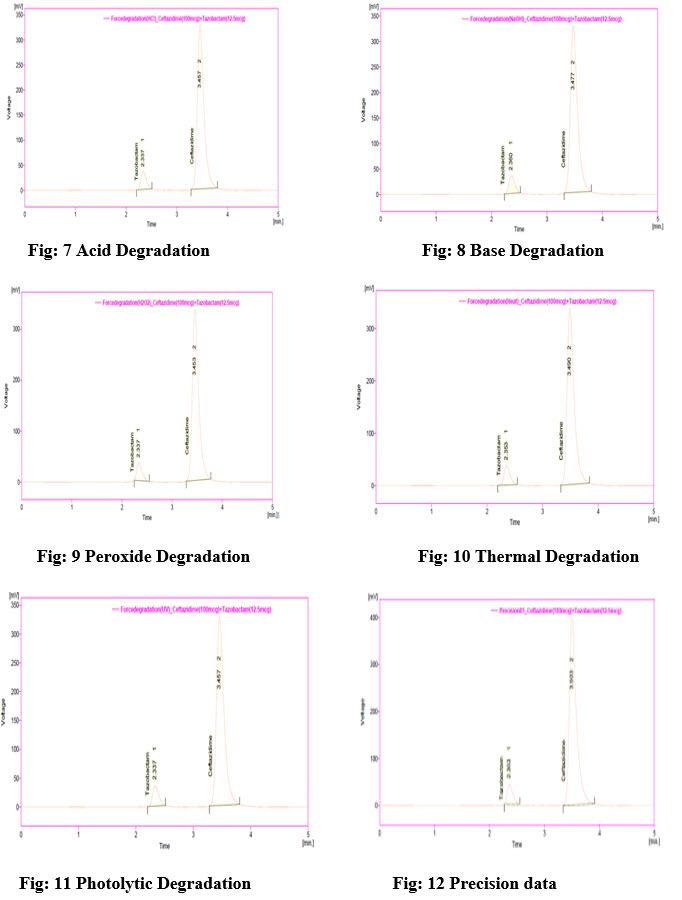
Acid and Base degradation:
Acid/base degradation was determined by taking 5ml of stock solution in 10ml volumetric flask and to this 2ml of 0.1N HCl/NaoH was added and sonicate for 5min, kept aside for 12hrs at room temperature. After 12hrs the solution was neutralized with 2ml of 0.1N HCL/NaoH then diluted with diluents to get a concentration of 10µg/ml solution.
Oxidative degradation:
Oxidative degradation was determined by taking 5ml of stock solution in 10ml volumetric flask and diluted up to the mark with 5% H2O2 and kept aside for 12hrs. After 12hrs the solution was diluted with diluents to get a concentration of 10µg/ml solution.
Thermal degradation:
Sample powder equivalent to 100mg of Ceftazidime and 12.5mg of Tazobactam was taken and kept in a controlled temperature oven at 800c for 12hrs. After 12hrs the powder was diluted with diluents to get a concentration of10µg/ml solution.
Photolytic degradation:
The Ceftazidime and Tazobactam powder and solutions of both were prepared and exposed to light to determine the irradiation of light on the stability of solution and powder form of drugs. Approximately 100mg of drug powder and 1mg/ml solution were spread on a glass dish in a layer that was less than 2mm thickness and were placed in a light cabinet and exposed to UV light for 12hrs. After 12hrs the samples are removed and diluted with diluents to get a concentration of10µg/ml solution and then injected.
The summary of results is tabulated in table no: 5 and figures are shown in figure no: 7,8,9,10,11.
DISSCUSSION:
Several trials has made until getting good peak resolution, acceptable plate count and tailing factor. Method was optimized and the retention times of Ceftazidime and Tazobactam was reported as 3.480 & 2.353.
SPECIFICITY:
The Chromatograms of Standard and Sample are identical with nearly same Retention time. There is no interference with blank and placebo to the drugs. Hence the proposed method was found to be specific.
LINEARITY:
From the Linearity data it was observed that the method was showing linearity in the concentration range of 60-140μg/ml for Ceftazidime and 7.5-17.5μg/ml for Tazobactam. Correlation coefficient was found to be 0.999 for both the compounds.
ACCURACY:
The recoveries of pure drug from the analyzed solution of formulation were 99.91 % for Ceftazidime pentahydrate and 100.84 % for Tazobactam sodium, which shows that the method was accurate.
PRECISION:
The %RSD for the sample chromatograms of method precision were found to be 0.39 & 0.81 for Ceftazidime and 0.23 &0.64 for Tazobactam. Hence it passes method precision.
ROBUSTNESS:
- All the system suitability parameters are within limits for variation in flow rate (±0.2 ml). Hence the allowable flow rate should be within 0.8 ml to 1.2 ml.
- All the system suitability parameters are within limits for variation (±2nm) in wavelength. Hence the allowable variation in wavelength is ± 2nm.
RUGGEDNESS:
Comparison of both the results obtained for two different Analysts shows that the method was rugged for Analyst-Analyst variability. The %RSD for intermediate precision for Ceftazidime was found to be 0.42 & 0.31 and for Tazobactam was found to be 0.74 & 0.26.
LOD & LOQ of Ceftazidime was found to be 0.03, 0.10and for Tazobactam was found to be 0.57, 1.72 respectively.
All the system suitability parameters are within in the limits when the drugs are subjected to stress conditions like acid, base peroxide, thermal and photolysis.
The results obtained were satisfactory and good agreement as per the ICH guidelines.
ACKNOWLEDGEMENT:
The authors thankful to Dr.Nanda Kishore Agarwal Professor, Department of Pharmaceutical Analysis and Quality Assurance for his valuable guidance and all the staff and non-teaching staff of Nimra College of Pharmacy for providing necessary facilities to carry out the research work.
The authors would like to thank beloved parents and all my well wishers, one and all who have helped me directly and indirectly in completing this project work.
CONCLUSION
Finally it concludes that all the parameters are within the limits and meet the acceptance criteria of ICH guidelines for method validation. The proposed method was simple, accurate, specific, precise, robust, rugged and economical. Hence this method is validated and can be used for routine and stability sample analysis.
FIGURES AND TABLES
Optimized chromatogram conditions for Ceftazidime and Tazobactam
Table no: 1
|
Column |
Hypersil BDS C18 (150*4.6mm,5µ) |
|
Mobile phase |
Phosphate Buffer pH 3.0:ACN:THF(60:35:05) |
|
Flow rate |
1.0 ml/ min |
|
Wavelength |
205 nm |
|
Injection volume |
20 ml |
|
Column temperature |
Ambient |
|
Run time |
8 min |
Table No: 2
Specificity Data for Ceftazidime and Tazobactam:
|
Standard Injection |
Retention time |
Area |
Theoretical Plates |
Retention time |
Area |
Theoretical Plates |
|
3.46 |
2910.42 |
3745 |
2.353 |
247.82 |
2254 |
|
|
3.50 |
3091.18 |
3817 |
2.357 |
285.2 |
2137 |
|
|
3.47 |
2943.91 |
3410 |
2.343 |
277.82 |
2210 |
|
|
Sample Injection
|
3.48 |
2984.39 |
3226 |
2.350 |
264.30 |
2125 |
|
3.48 |
2996.31 |
3781 |
2.352 |
262.89 |
2248 |
|
|
3.47 |
2989.06 |
3410 |
2.360 |
263.40 |
2143 |
|
|
Blank injection |
- |
- |
- |
- |
- |
- |
Linearity data for Ceftazidime and Tazobactam
Table .No: 3
|
S.NO |
For Ceftazidime |
For Tazobactam |
||||
|
Mcg/ml |
Area |
Rt |
Mcg/ml |
Area |
Rt |
|
|
1. |
60 |
1847.225 |
3.470 |
7.5 |
163.031 |
2.337 |
|
80 |
2526.511 |
3.463 |
10 |
223.261 |
2.330 |
|
|
100 |
3072.796 |
3.490 |
12.5 |
276.082 |
2.353 |
|
|
120 |
3793.229 |
3.467 |
15 |
337.177 |
2.336 |
|
|
140 |
4341.245 |
3.453 |
17.5 |
394.041 |
2.323 |
|
|
Slope |
31.27 |
|
Slope |
23.037 |
|
|
|
Correlation coefficient |
0.99913 |
|
Correlation Coefficient |
0.999811 |
|
|
|
Intercept |
11.17 |
|
Intercept |
9.249 |
|
|
Summary of validation parameters:
Table No: 4
|
S.NO |
Parameter |
Ceftazidime |
Tazobactam |
||
|
1. |
Linearity |
60-140µg/ml |
7.5-17.5µg/ml |
||
|
2. |
Precision(% RSD) |
0.39 |
0.81 |
0.23 |
0.64 |
|
3. |
Accuracy |
99.91% |
100.84% |
||
|
4. |
LOD & LOQ |
0.03,0.10 |
0.57,1.72 |
||
|
5. |
Assay |
99.38% |
99.26% |
||
|
6. |
Ruggednes (%RSD) |
0.42, 0.31 |
0.74,0.26 |
||
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Forced degradation data for Ceftazidime and Tazobactam:
Table No: 5
|
Stress Condition |
Time(hrs) |
Retention |
Time(hrs) |
Retention Time |
|
As such |
12hrs |
3.490 |
12hrs |
2.353 |
|
Acid Hydrolysis (0.1 N, at RT) |
12hrs |
3.457 |
12hrs |
2.337 |
|
Base Hydrolysis (0.1N at RT) |
12hrs |
3.477 |
12hrs |
2.360 |
|
Oxidation (5% H2O2 at RT) |
12hrs |
3.453 |
12hrs |
2.353 |
|
Photolysis(UV Light and sunlight) |
12hrs |
3.457 |
12hrs |
2.337 |
|
Thermal (at 800c) |
12hrs |
3.490 |
12hrs |
2.353 |
REFERENCES:
1.Nanda R.K, Ashwini Shelke.V. Development And Validation Of HPTLC Method For The Simultaneous Estimation Of Ceftazidime Sodium And Tazobactam Sodium In Marketed Formulation. International Journal of ChemTech Research; Vol.4, Oct-Dec 2012, pg 1701-1707.
2.Nanda R.K, Ashwini Shelke.V. UV Spectrophotometric Methods For Simultaneous Estimation Of Ceftazidime Sodium And Tazobactam Sodium In Dry Powder Injection. International Journal of ChemTech Research; Vol.4, Oct-Dec 2012, pg 1778-1785.
3.Rama Krishna Veni.P, Sharmila.N, Narayana K.J.P, Hari Babu B, Satyanarayana P.V.V. Simultaneous Determination Of Piperacillin And Tazobactam In Pharmaceutical Formulations By RP-HPLC Method. Journal of Pharmacy Research (7), Nov-Feb2012, pg.no 127 – 131.
4.Lakshmanarao.A, Sai Krishna.K, Kiran Kumar.CH and Raja.T. Simultaneous Determination Of Piperacillin And Tazobactum In Bulk And Pharmaceutical Dosage Forms By RP-HPLC, International Journal of Pharmacy and Pharmaceutical Sciences, vol 3,2011, pg.no 134-36.
5.Shaikh K.A. Steven A. Signs, Thomas M. File Method Development And Validation For Third Generation Cephalosporin By UV-Visible Spectrophotometer, IRJP, vol-2, 2011, pg.no 222-229.
6.Senem Sanli, Nurullah Sanli, Simultaneous Estimation of Ceftazidime and Ceftizoxime in Pharmaceutical Formulations by HPLC Method, Chromatographia, Vol-4, 2011, pg.no 7-8.
7.Narendra Kumar.R, Nageswara Rao.G, Naidu.P.Y, Stability Indicating Fast LC Method For Determination Of Ceftriaxone And Tazobactam For Injection Related Substances In Bulk And Pharmaceutical Formulation. IJABPT, Volume: I, May-July 2010, pg.no 546-48.
8.Gandhimathi.M, Saravanakumar.M, Ravi.T.K. Validated Ion Pair HPLC Method For Simultaneous Estimation Of Ceftriaxone Sodium And Tazobactum Sodium In Dosage Form. International Journal of Pharma and Bio Sciences; Vol.1, Oct-Dec.2010, pg.no 17-22.
9.Hiremath B, Mruthyunjayaswamy B.H.Development And Validation Of Spectrophotometric Methods For Determination Of Ceftazidime In Pharmaceutical Dosage Forms. PubMed, Acta Pharm. vol-3, 2008 Sep, pg.no 275-85.
10.Arun.K,C. Saravanan, R.Balachandar, M.V.Kumuthavalli, B.Jayakar, UV- Spectrophotometric Determination Of Ceftazidime In Pure And Pharmaceutical Formulation, J.Chem.Pharm. Res. Vol-2, 2010, pg.no 424-431.
11.Moreno Ade H, Salgado HR., Rapid And Selective UV Spectrophotometric Method For The Analysis Of Ceftazidime, PubMed, J AOAC Int. vol-92, May-Jun2009, pg.no 820-823.
12.Moreno Ade H, Salgado HR., Development Of A New High-Performance Liquid Chromatographic Method For The Determination Of Ceftazidime. PubMed, JAOAC Int., vol-4, Jul-Aug 2008, pg.no739-743.
13.Beckett AH, Stenlake JB. PRACTICAL PHARMACEUTICAL CHEMISTRY. Vol. II. CBS Publisher and Distributors, New Delhi, 1986, pg.no 13-17.
14.Skoog, DA Holler, FJ Nieman. T.A. PRINCIPLES OF INSTRUMENTAL ANALYSIS, Fifth edition. Thomson Brooks/Cole. 2009, pg.no 835-890.
15.Sharma BK. INSTRUMENTAL METHODS OF CHEMICAL ANALYSIS, Eleventh edition, Goel Publishing House, Meerut. 1991, pg.no 1-9.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









