About Authors:
RAVIKUMAR R. PATEL1, Dr. JAYVADAN K. PATEL2
1 Research Scholar, Jodhpur National University, Jodhpur
2 Principal and Professor, Nootan Pharmacy College, Visnagar, Mehsana, Gujarat.
AIM OF PRESENT INVESTIGATION
Now a day most of the pharmaceutical scientists are involved in developing an ideal drug delivery system with an objective of not only curing the disease but also to achieve patient compliance. Approximately 50 % of the drug delivery system available in market is oral drug delivery system. Conventional oral dosage forms provide a specific drug concentration in systemic circulation without offering any control over drug delivery. Over the past three decades, the pursuit and exploration of devices designed to be retained in the upper part of gastrointestinal tract has advanced consistently in terms of technology and diversity, encompassing a variety of systems and devices such as floating systems.
[adsense:336x280:8701650588]
Hydrochlorothiazide is a thiazide diuretic commonly prescribed for treatment of hypertension, congestive heart failure and edema. It is mostly absorbed from the duodenum and the first part of the jejunum1. Bioavailability of hydrochlorothiazide was enhanced when given with food through delaying of gastric emptying in both cases. Bioavailability was lower from SR formulation such as pellets compared to an IR tablet, because the SR formulation passed the drug absorption sites before from completed the drug release. These studies support the absorption window theory for hydrochlorothiazide and therefore a higher bioavailability of the drug in a GRD can be expected if there is a prolonged gastric residence time1. The above information supports the theory that hydrochlorothiazide has an absorption window, which makes it a good candidate for intragastric floating drug delivery system.
Gastro retentive systems extend significantly the period of time over which the drugs may be released. Thus not only prolong dosing interval but also increase patient compliance beyond the level of existing controlled release dosage forms. Most of the old age population in India suffers from hypertension, heart diseases, and angina pectoris. Antihypertensive agents and calcium channel blockers are mainly used to treat those geriatric patients. These cardiovascular drugs suffer from low bioavailability problems, short half life due to extensive first pass metabolism therefore short duration of action; this makes discomfort to old age people. Recent development in novel drug delivery system, a new approach Gastro retention is solution to these problems of cardiovascular drugs. Floating microspheres have a bulk density less than gastric fluid and so remain buoyant in the stomach for a prolonged period of time. While the system floats over gastric contents, the drug is released slowly at the desired rate from the system, therefore improving bioavailability of drug and better control over the fluctuations in plasma drug concentration. Thus it not only reduces dosing frequency but also reduces side effects like gastric irritation and major problem like dose dumping thereby greatly increasing patient compliance.
The gastroretentive drug delivery system can be retained in the stomach and assists in improving the oral sustained delivery of the drugs that have and absorption window in a particular region of the GI tract, these systems help in continuous releasing the drug before it reaches the absorption window, thus ensuring optimal bioavailability. Several approaches are currently used to prolong gastric retention time .These include floating drug delivery system, swelling and expanding systems, polymeric bioadhesive system, high –density and other delayed gastric-emptying devices2. The principal of buoyant preparation offers a simple and practical approach to achieve increased gastric residence time for dosage from and sustained drug release3. The present investigation describes the formulation development of an intragastric floating drug delivery system of Hydrochlorothiazide.
The objective of the present study is to develop suitable gastroretentive floating microspheres of Hydrochlorothiazide and to study release kinetics of drug with a view to reduce the dose frequency and to achieve a controlled drug release via gastric retention with improved bioavailability.
Reference Id: PHARMATUTOR-ART-1218
INTRODUCTION
2.1 INTRODUCTION TO FLOATING MICROSPHERES
Microspheres can be defined as solid, approximately spherical particles ranging in size from 1 to 1000 micrometer. The Microspheres are characteristically free flowing powders consisting of proteins or synthetic polymers, which are biodegradable in nature. Solid biodegradable microspheres incorporating a drug dispersed or dissolved throughout particle matrix have the potential for controlled release of drugs4. Microspheres are small in size and therefore have large surface to volume ratios. The concept of incorporating microscopic quantities of materials within microspheres dates back to the 1930s and to the work of Bungerberg de joing and co-workers on the entrapment of substances within coacervates. The potential uses of microspheres in the pharmaceutical have been considered since the 1960’s and have a number of applications. The use of microspheres in pharmaceuticals have a number of advantages Viz., Taste and odor masking, conversion of oils and other liquids to solids for ease of handling, protection of drugs against environment (moisture, heat, light and oxidation), separation of incompatible materials, to improve flow of powders, production of sustained release, controlled release and targeted medications. The most important physico-chemical characteristics that may be controlled in microspheres manufacture are; particle size and distribution, polymer molecular weight, ratio of drug to polymer, total mass of drug and polymer.5, 6
A number of different substances both biodegradable as well as non-biodegradable have been investigated for the preparation of microspheres; these materials include polymers of natural origin or synthetic origin and also modified natural substances. A range of microspheres prepared using both hydrophilic and hydrophobic polymers. Hydrophilic polymers includes gelatin, agar, egg albumin, starch, chitosan, cellulose derivatives; HPMC, DEAE cellulose. Hydrophobic polymers include ethyl cellulose, polylactic acid, PMMA, acrylic acid esters etc7. The number of polymers and range of formulation variables available to control the rate of drug release from controlled release devices are broad. Selection among these variables is based upon the desired release rate and duration, physical and chemical properties of the drug and the intended site of administration. In general polymers are selected from one of several classes as follows:
[adsense:468x15:2204050025]
Non-Biodegradable Hydrophobic Polymers:
These materials are inert in the environment of use, are eliminated or extracted intact from the site of administration, and serve essentially as a rate limiting barrier to the transport and release of drug from the device. Common examples of such materials include polyethylene vinyl acetate (EVA), Polydimethyl siloxane (PDS), Polyether urethane (PEU), Ethyl cellulose (EC), Cellulose acetate (CA), Polyethylene (PE) and Polyvinyl chloride (PVC), Acrycoat, Eudragit S etc.8, 9
Hydrogels: These polymers swell but do not dissolve when brought in contact with water. They are obtained from natural or synthetic sources. As with the hydrophobic polymers, hydrogels are inert, removed intact from the site of administration, and function by forming a rate limiting barrier to the transport and release of drugs. Common examples include polyhydroxy ethyl methyl acrylate (PHEMA), cross-linked poly vinyl alcohol (PVA), cross linked poly vinyl pyrrolidone (PVP), poly acryl amide etc.10
Soluble polymers:These are moderate molecular weight (less than 75,000 Daltons) uncross linked polymers that dissolve in water. The rate of dissolution decreases with increasing molecular weight. These materials can be used alone or in combination with hydrophobic polymers to provide devices that slowly erode over time. Examples include polyethylene glycol (PEG), uncross linked poly vinyl alcohol or poly vinyl pyrrolidone, hydroxyl propyl methyl cellulose (Methocel) and copolymers of metha acrylic acid and acrylic acid methyl ester (Eudragit L) etc.
Biodegradable polymers:These materials also slowly disappear from the site of administration; however it occurs in response to a chemical reaction such as hydrolysis. Examples of biodegradable polymers include polylactic acid (PLA), poly glycolic acid (PGA), Polycaprolactone (PCL) and several generic classes such as the poly anhydrides and poly orthoesters.11
The range of techniques for the preparation of microspheres offers a variety of opportunities to control aspects of drug administration. Microspheres can be prepared by:
Single Emulsion Technique, Double Emulsion Technique, Polymerization technique, Phase Separation coacervation technique, Spray Drying and Spray Congealing, Solvent Extraction12
Floating microspheres are gastro-retentive drug delivery systems based on non-effervescentapproach. Floating microspheres are in strict sense, spherical empty particles without core. These microspheres are also termed as “Microballoons” due to its characteristic internal hollow structure and excellent floatability in vitro.
Gastro-retentive floating microspheres are low-density systems that have sufficient buoyancy to float over gastric contents and remain in stomach for prolonged period. As the system floats over gastric contents, the drug is released slowly at desired rate resulting in increased gastric retention with reduced fluctuations in plasma drug concentration 13.
Advantages of Floating Microspheres:
• Improves patient compliance by decreasing dosing frequency.
• Bioavailability enhances despite first pass effect because fluctuations in plasma drug concentration is avoided, a desirable plasma drug concentration is maintained by continuous drug release.
• Better therapeutic effect of short half-life drugs can be achieved.
• Gastric retention time is increased because of buoyancy.
• Drug releases in controlled manner for prolonged period.
• Site-specific drug delivery to stomach can be achieved.
• Enhanced absorption of drugs which solubilizes only in stomach.
• Superior to single unit floating dosage forms as such microspheres releases drug uniformly and there is no risk of dose dumping. 12
• Avoidance of gastric irritation, because of sustained release effect, floatability and uniform release of drug through multiparticulate system.
• The flow characteristics and packability of the resultant microballoons are much improved when compared with the raw crystals of the drug.
• Drug targeting to stomach can be attractive for several other reasons 14
For the weakly basic drugs with poor solubility in the basic environment, the floating systems may avoid any chance of solubility to become the rate-limiting step in the release by restricting the drug to the stomach.
Any solute released in the stomach will empty along with the fluids such that the whole surface of the small intestine is available for absorption. This is particularly useful when an absorption window exists in the proximal small intestine15.
The positioned gastric release is useful for all substances intended to produce a lasting local action onto the gastroduodenal wall.
Limitations of floating Microspheres
• The major disadvantage of floating systems is requirement of sufficiently high levels of fluids in the stomach for the drug delivery16.
• The dosage form should be administered with a minimum of glass full of water8.
• Floating system is not feasible for those drugs that have solubility or stability problems in gastric fluids.
• Single unit floating capsules or tablets are associated with an “all or none concept,” but this can be overcome by formulating multiple unit systems like floating microspheres or microballoons17.
• Drugs that irritate the mucosa, those that have multiple absorption sites in the gastrointestinal tract, and those that are not stable at gastric pH are not suitable candidates to be formulated as floating dosage forms.13
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Preliminary Screening of the formulations
Floating microspheres loaded with Verapamil hydrochloride were prepared by Emulsion solvent evaporation method.26, 27 Formulations were formulated using different polymers Kollidone SR, cellulose acetate, Acrycoat S 100, Methocel K4M, Methocel K15M, Methocel K100M. Drug and polymer in proportion 1:2, (drug: polymer) were dissolved in organic solvent (Ethanol31, 32 and Acetone28, 29). This clear solution was poured slowly as a thin stream in oil phase; about 100 ml of liquid paraffine solution with continuous stirring at a speed of 500 rpm using remi stirrer at room temperature until complete evaporation of solvent took place. The floating microspheres were collected by decantation, while the non floating microspheres were discarded along with any polymer precipitates. The microspheres were then dried overnight at 400C. The microspheres were weighed and stored in desiccators until further analysis. Overall six formulations were formulated using different polymers, Methocel K4M (code F1), Methocel K15 (code F2) and Methocel K100M (code F3), Cellulose acetate (code F4), Acrycoat S100 (code F5), Kollidone SR (code F6) as shown in Table 1.
Table 1. Composition of floating microspheres of Hydrochlorothiazide
(Preliminary Screening of the formulations)
|
Sr. No. |
Formulation code |
Drug : Polymer Ratio |
Organic solvent |
Continuous Phase |
|
1 |
F1 |
1:2 |
Ethanol |
Liquid paraffin containing 0.01% tween 80 |
|
2 |
F2 |
1:2 |
Ethanol |
Liquid paraffin containing 0.01% tween 80 |
|
3 |
F3 |
1:2 |
Ethanol |
Liquid paraffin containing 0.01% tween 80 |
|
4 |
F4 |
1:2 |
Acetone |
Liquid paraffin containing 0.01% tween 80 |
|
5 |
F5 |
1:2 |
Ethanol |
Liquid paraffin containing 0.01% tween 80 |
|
6 |
F6 |
1:2 |
Acetone |
Liquid paraffin containing 0.01% tween 80 |
CHARACTERIZATION OF PREPARED MICROSPHERES
(Preliminary Screening of the formulations)
Micromeritics properties of floating microspheres:30
1.Particle size determination : The particle size was determined using an optical microscope under regular polarized light, and mean particle size was calculated by measuring 100 particles with the help of a calibrated oculometer. Results shown in Table 2.
2.Bulk density: Bulk density was determined by three tap method, after filling the weighed quantity of microspheres in a graduated cylinder, the volume occupied by microspheres was determined..
3. Tapped density: The tapping method was used to calculate tapped densities. The volume of weighed quantity of microspheres was determined after 100 taps as well as 1000 taps using tapped density apparatus.
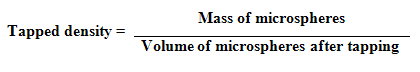
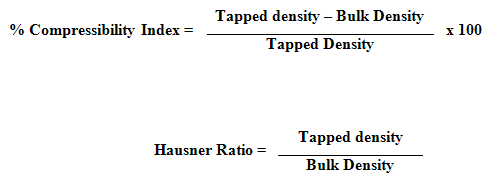
4. Compressibility Index and Hausner Ratio : Compressibility index and hausner ratio was calculated from the values of bulk density and tapped density by using following formulas :
5. Angle of Repose: The angle of repose θ of the microspheres, which measures the resistance to particle flow, was calculated as tan θ = 2 H / D
Where 2H/D is the surface area of the free standing height of the microspheres heap that is formed after making the microspheres flow from the glass funnel. Results shown in Table 2.
6. Yield of Microspheres: The prepared microspheres were collected and weighed. The measured weight was divided by the total amount of all non-volatile components which were used for the preparation of the microspheres. Results shown in Table 3.
% Yield = (Actual weight of product / Total weight of excipients and drug) x 100
7. DEE (Drug Entrapment Efficiency): 1 gm of formulation (microspheres) was taken for evaluation. The amount of drug entrapped was estimated by crushing the microspheres and extracting with aliquots of 0.1 N HCL repeatedly. The extract was transferred to a 100 ml volumetric flask and the volume was made up using 0.1 N HCl. The solution was filtered and the absorbance was measured after suitable dilution spectrophotometrically at 278 nm against appropriate blank. The amount of drug entrapped in the microspheres was calculated by the following formula:
DEE = (Amount of drug actually present / Theoretical drug load expected) x 100
8. In Vitro Evaluation of Floating Ability: 33, 34
In vitro floatability studies of floating microspheres were carried out using USP apparatus II. To assess the floating Properties, the microspheres (300 mg) were placed in 0.1 N hydrochloric acid (500 ml) containing 1 % Tween 80 surfactant to simulate gastric conditions. The use of 1 % Tween was to account for the wetting effect of the natural surface active agents such as phospholipids in the GIT and so excluding the floating due to non-wetted surfaces. A paddle rotating at 100 rpm agitated the medium. Each fraction of microspheres floating on the surface and those settled down were collected at 12 hr. The collected samples were weighed after drying. Results shown in Table 3.
The buoyancy was calculated as
% floating microspheres = QF / (QF + QS) x 100
Where QF and QS are weights of the floating and the settled microspheres respectively.
Table 2. Micromeritics Properties of Floating Microspheres
|
Formulation Code |
Angle of repose ±SDa |
Carr’s Index (%) ±SDa |
Hausner ratio ±SDa |
Mean Particle Size (µm)±SDa |
|
F1 |
23.83º ±0.311 |
13.96 ±4.921 |
1.16 ±0.071 |
362.77 ±3.35 |
|
F2 |
22.63º ±0.601 |
15.88 ±0.566 |
1.20 ±0.007 |
255.81 ±2.29 |
|
F3 |
29.86º ±0.071 |
19.92 ±1.428 |
1.26 ±0.017 |
466.64 ±3.73 |
|
F4 |
31.42º ±0.035 |
29.43 ±0.239 |
1.27 ±0.042 |
642.66 ±5.86 |
|
F5 |
29.89º ±0.559 |
29.24 ± 1.598 |
1.28 ±0.021 |
596.73 ±5.33 |
|
F6 |
33.42º ± 0.672 |
32.30 ± 0.629 |
1.29 ±0.007 |
668.79 ±6.93 |
a Mean ± standard deviation
Table 3. Characterization of prepared floating microspheres (preliminary batches)
|
Formulation Code |
% Yield ± SDa |
% Drug Entrapped ±SDa |
% Buoyancy at 12 h ±SD a |
|
F1 |
86.90 ± 3.70 |
85.6 ± 2.07 |
74.9 ±4.25 |
|
F2 |
77.18 ± 1.10 |
82.4 ± 2.75 |
61.8 ±2.35 |
|
F3 |
58.62 ± 4.09 |
76.4 ± 3.50 |
55.6 ±6.84 |
|
F4 |
36.86 ± 6.32 |
52.56 ± 1.67 |
46.20 ± 4.21 |
|
F5 |
39.43 ± 5.85 |
54.47 ± 2.61 |
48.26 ± 3.56 |
|
F6 |
51.42 ± 7.63 |
58.56 ± 1.67 |
49.73 ± 2.46 |
a Mean ± standard deviation
From the results of evaluation of the floating microspheres for preliminary screening it was found that the microspheres prepared with cellulose acetate, Acrycoat and Eudragit S 100 were not showing the satisfactory results. The micromeritics properties of the prepared floating microspheres with cellulose acetate, Acrycoat and Eudragit S 100 shown that the flow properties of the microspheres were poor. The value of angle of repose of formulation F1, F2 and F3 were found to be 33.42º ± 0.672, 31.42º ±0.035 and 29.89º ±0.559 respectively which indicates poor flow properties. % yield of the formulation F1, F2 and F3 were found to be 36.86 ± 6.32, 39.43 ± 5.85 and 51.42 ± 7.63 respectively. Drug entrapment efficiency of the formulation F1, F2 and F3 were found to be 52.56 ± 1.67, 54.47 ± 2.61 and 58.56 ± 1.67 respectively. % Buoyancy at 12 h of the formulation F1, F2 and F3 were found to be 46.20 ± 4.21, 48.26 ± 3.56 and 49.73 ± 2.46 respectively. % yield, Drug entrapment efficiency and % Buoyancy at 12 h of the formulation F1, F2 and F3 were found to be comparatively less than formulation F4, F5 and F6. So formulation F4, F5 and F6 were selected for further studies with modifications.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4.3.2.6 SELECTION OF THE FORMULATIONS FOR FURTHER STUDIES
The screening of microspheres formulations were based on different physicochemical and evaluation parameters. The optimistic formulations from above all are F4, F5 and F6. These formulations shows satisfactory results of different physicochemical parameters and evaluation parameters therefore modifications in these three formulations were made by varying the drug: polymer ratio, solvent systems and change in aqueous phase also investigated.
4.3.2.7 OPTIMIZATION OF THE SELECTED FORMULATIONS
Optimization of the formulations F4, F5 and F6:
Modification of all above formulations were affected by preparing the microspheres using different ratio of drug and polymer, combine effect of solvent system and change in aqueous phase also investigated. The floating microspheres were prepared according to the method given in the section 4.3.2.4. They are designated as K1, K2, K3, A1, A2, A3, C1, C2, and C3. The detailed composition was given in the Table 4.8.
Table 4.8 Compositions of optimized formulations of floating microspheres
|
Sr. No. |
Formulation code |
Drug: Ratio |
Organic solvent system [1:1] |
Continuous Phase |
|
1 |
K1
|
1:1 |
Ethyl acetate: acetone
|
Liquid paraffin containing 0.01% tween 80 |
|
2 |
K2
|
1:2 |
Ethyl acetate: acetone |
Liquid paraffin containing 0.01% tween 80 |
|
3 |
K3
|
1:1 |
Ethyl acetate: acetone |
Liquid paraffin containing 0.01% tween 80 |
|
4 |
A1
|
1:1 |
Dichloromethane: ethanol |
Liquid paraffin containing 0.01% tween 80 |
|
5 |
A2
|
1:2 |
Dichloromethane: ethanol |
Liquid paraffin containing 0.01% tween 80 |
|
6 |
A3
|
1:1 |
Dichloromethane: ethanol |
Liquid paraffin containing 0.01% tween 80 |
|
7 |
C1
|
1:1 |
Ethyl acetate: acetone |
Liquid paraffin containing 0.01% tween 80 |
|
8 |
C2
|
1:2 |
Ethyl acetate: acetone |
Liquid paraffin containing 0.01% tween 80 |
|
9 |
C3
|
1:1 |
Dichloromethane: ethanol |
Liquid paraffin containing 0.01% tween 80 |
NOTE:
- Formulations K1, K2 and K3 containing Kollidone SR.
- Formulations A1, A2 and A3 containing Acrycoat S 100.
- Formulations C1, C2 and C3 containing Cellulose acetate.
4.3.2.8 CHARACTERIZATION OF OPTIMIZED FORMULATIONS
Micromeritics propertiesand evaluations like Yield of microspheres, Drug entrapment efficiency and in vitro floating ability of the optimized formulations were done according to the methods given in the section 4.3.2.5. Results are given in Table 4.9 and Table 4.10.
Table 4.9 Micromeritics properties of floating microspheres.
|
Formulation code |
Mean Particle Size (µm)±SD |
Flow Properties |
||
|
% Compressibility ±SD |
Hausner Ratio ±SD |
Angle of Repose ± SD |
||
|
K1 |
344.70 ±3.81 |
13.86 ±0.26 |
1.17 ±0.041 |
25.42 ±0.67 |
|
K2 |
360.75 ±3.30 |
14.30 ±0.62 |
1.19 ±0.007 |
24.42 ±0.03 |
|
K3 |
382.50 ±3.09 |
16.43 ±0.23 |
1.24 ±0.017 |
23.89 ±0.55 |
|
A1 |
252.45 ±4.63 |
16.25 ±1.59 |
1.24 ±0.028 |
22.83 ±0.31 |
|
A2 |
253.80 ±2.27 |
15.86 ±2.92 |
1.21 ±0.028 |
22.63 ±0.60 |
|
A3 |
279.00 ±1.27 |
17.78 ±0.56 |
1.26 ±0.07 |
29.88 ±0.07 |
|
C1 |
418.95±8.81 |
17.92 ±1.42 |
1.26 ±0.016 |
29.46 ±0.58 |
|
C2 |
463.64 ±3.68 |
19.36 ±2.10 |
1.27 ±0.017 |
30.23 ±0.28 |
|
C3 |
411.61 ±4.86 |
21.55 ±1.88 |
1.29 ±0.041 |
30.48 ±0.68 |
Table 4.10 Characteristics of Hydrochlorothiazide floating microspheres.
|
Formulation code |
% Yield |
% Drug Entrapped |
% Buoyancy at 12 h ±SD |
|
K1 |
97.40 |
83.8 % |
72.2 ±2.687 |
|
K2 |
84.85 |
84.7 % |
73.8 ±3.253 |
|
K3 |
87.16 |
82.6 % |
68.6 ±2.121 |
|
A1 |
77.14 |
82.9 % |
62.7 ±0.849 |
|
A2 |
75.15 |
81.3 % |
61.8 ±1.273 |
|
A3 |
73.59 |
80.6 % |
63.6 ±0.636 |
|
C1 |
44.93 |
75.6 % |
47.0 ± 1.344 |
|
C2 |
55.60 |
77.8 % |
50.6 ±0.849 |
|
C3 |
68.00 |
72.9 % |
53.9 ±1.273 |
4.3.2.8.1 In vitro drug release studies19, 35
The drug release studies were carried out using six basket dissolution apparatus USP type II. The microspheres were placed in a non reacting mesh that had a smaller mesh size than the microspheres. The mesh was tied with a nylon thread to avoid the escape of any microspheres. The dissolution medium used was 900 ml of 0.1 N hydrochloric acid at 37°C. At specific time intervals, 5 ml aliquots were withdrawn and analyzed by UV spectrophotometer at the respective lmax value 271 nm after suitable dilution against suitable blank. The withdrawn volume was replaced with an equal volume of fresh 0.1 N hydrochloric acid. The dissolution data of all nine formulations are given in Table 4.11 to 4.19.
Table 4.11 Dissolution data of formulation K1
|
Sr. No. |
Time(h) |
Root t |
Cumu. % release |
Log Cumu. % release |
Cumu. % retained |
Log Cumu. % retained |
|
1 |
1 |
1.000 |
30.39 |
1.483 |
69.61 |
1.843 |
|
2 |
2 |
1.414 |
36.95 |
1.568 |
63.05 |
1.800 |
|
3 |
3 |
1.732 |
41.96 |
1.623 |
58.04 |
1.764 |
|
4 |
4 |
2.000 |
48.15 |
1.683 |
51.85 |
1.715 |
|
5 |
5 |
2.236 |
53.13 |
1.725 |
46.87 |
1.671 |
|
6 |
6 |
2.449 |
56.99 |
1.756 |
43.01 |
1.634 |
|
7 |
7 |
2.645 |
60.16 |
1.779 |
39.84 |
1.600 |
|
8 |
8 |
2.828 |
68.03 |
1.833 |
31.97 |
1.505 |
|
9 |
9 |
3.000 |
75.49 |
1.878 |
24.51 |
1.389 |
|
10 |
10 |
3.162 |
83.31 |
1.921 |
16.69 |
1.222 |
|
11 |
11 |
3.316 |
87.18 |
1.940 |
12.82 |
1.108 |
|
12 |
12 |
3.464 |
94.75 |
1.977 |
5.25 |
0.720 |
Table 4.12 Dissolution data of formulation K2
|
Sr. No. |
Time(h) |
Root t |
Cumu. % release |
Log Cumu. % release |
Cumu. % retained |
Log Cumu. % retained |
|
1 |
1 |
1.000 |
29.14 |
1.464 |
70.86 |
1.850 |
|
2 |
2 |
1.414 |
35.82 |
1.554 |
64.18 |
1.807 |
|
3 |
3 |
1.732 |
40.69 |
1.609 |
59.31 |
1.773 |
|
4 |
4 |
2.000 |
47.98 |
1.681 |
52.02 |
1.716 |
|
5 |
5 |
2.236 |
54.16 |
1.734 |
45.84 |
1.661 |
|
6 |
6 |
2.449 |
56.26 |
1.750 |
43.74 |
1.641 |
|
7 |
7 |
2.645 |
59.94 |
1.778 |
40.06 |
1.603 |
|
8 |
8 |
2.828 |
68.12 |
1.833 |
31.88 |
1.504 |
|
9 |
9 |
3.000 |
75.14 |
1.876 |
24.86 |
1.396 |
|
10 |
10 |
3.162 |
81.2 |
1.910 |
18.8 |
1.274 |
|
11 |
11 |
3.316 |
85.58 |
1.932 |
14.42 |
1.159 |
|
12 |
12 |
3.464 |
89.13 |
1.950 |
10.87 |
1.036 |
Table 4.13 Dissolution data of formulation K3
|
Sr. No. |
Time(h) |
Root t |
Cumu. % release |
Log Cumu. % release |
Cumu. % retained |
Log Cumu. % retained |
|
1 |
1 |
1.000 |
31.02 |
1.492 |
68.98 |
1.839 |
|
2 |
2 |
1.414 |
34.22 |
1.534 |
65.78 |
1.818 |
|
3 |
3 |
1.732 |
39.56 |
1.597 |
60.44 |
1.781 |
|
4 |
4 |
2.000 |
48.12 |
1.682 |
51.88 |
1.715 |
|
5 |
5 |
2.236 |
54.6 |
1.737 |
45.4 |
1.657 |
|
6 |
6 |
2.449 |
55.36 |
1.743 |
44.64 |
1.650 |
|
7 |
7 |
2.645 |
59.68 |
1.776 |
40.32 |
1.606 |
|
8 |
8 |
2.828 |
68.32 |
1.835 |
31.68 |
1.501 |
|
9 |
9 |
3.000 |
75.13 |
1.876 |
24.87 |
1.396 |
|
10 |
10 |
3.162 |
80.86 |
1.908 |
19.14 |
1.282 |
|
11 |
11 |
3.316 |
82.38 |
1.916 |
17.62 |
1.246 |
|
12 |
12 |
3.464 |
84.29 |
1.926 |
15.71 |
1.196 |
Table 4.14 Dissolution data of formulation C1
|
Sr. No. |
Time(h) |
Root t |
Cumu. % release |
Log Cumu. % release |
Cumu. % retained |
Log Cumu. % retained |
|
1 |
1 |
1.000 |
34.993 |
1.544 |
65.007 |
1.813 |
|
2 |
2 |
1.414 |
43.498 |
1.638 |
56.502 |
1.752 |
|
3 |
3 |
1.732 |
52.359 |
1.719 |
47.641 |
1.678 |
|
4 |
4 |
2.000 |
61.038 |
1.786 |
38.962 |
1.591 |
|
5 |
5 |
2.236 |
69.143 |
1.840 |
30.857 |
1.489 |
|
6 |
6 |
2.449 |
78.613 |
1.895 |
21.387 |
1.330 |
|
7 |
7 |
2.645 |
84.290 |
1.926 |
15.710 |
1.196 |
|
8 |
8 |
2.828 |
89.130 |
1.950 |
10.870 |
1.036 |
Table 4.15 Dissolution data of formulation C2
|
Sr. No. |
Time(h) |
Root t |
Cumu. % release |
Log Cumu. % release |
Cumu. % retained |
Log Cumu. % retained |
|
1 |
1 |
1.000 |
35.373 |
1.549 |
64.627 |
1.810 |
|
2 |
2 |
1.414 |
40.576 |
1.608 |
59.424 |
1.774 |
|
3 |
3 |
1.732 |
46.242 |
1.665 |
53.758 |
1.730 |
|
4 |
4 |
2.000 |
55.354 |
1.743 |
44.646 |
1.650 |
|
5 |
5 |
2.236 |
63.183 |
1.801 |
36.817 |
1.566 |
|
6 |
6 |
2.449 |
68.243 |
1.834 |
31.757 |
1.502 |
|
7 |
7 |
2.645 |
77.51 |
1.889 |
22.490 |
1.352 |
|
8 |
8 |
2.828 |
85.49 |
1.932 |
14.510 |
1.162 |
|
9 |
9 |
3.000 |
90.35 |
1.956 |
9.650 |
0.985 |
Table 4.16 Dissolution data of formulation C3
|
Sr. No. |
Time(h) |
Root t |
Cumu. % release |
Log Cumu. % release |
Cumu. % retained |
Log Cumu. % retained |
|
1 |
1 |
1.000 |
28.088 |
1.449 |
71.912 |
1.857 |
|
2 |
2 |
1.414 |
34.27 |
1.535 |
65.730 |
1.818 |
|
3 |
3 |
1.732 |
38.434 |
1.585 |
61.566 |
1.789 |
|
4 |
4 |
2.000 |
47.923 |
1.681 |
52.077 |
1.717 |
|
5 |
5 |
2.236 |
54.062 |
1.733 |
45.938 |
1.662 |
|
6 |
6 |
2.449 |
57.192 |
1.757 |
42.808 |
1.632 |
|
7 |
7 |
2.645 |
67.656 |
1.830 |
32.344 |
1.510 |
|
8 |
8 |
2.828 |
73.578 |
1.867 |
26.422 |
1.422 |
|
9 |
9 |
3.000 |
78.142 |
1.893 |
21.858 |
1.340 |
|
10 |
10 |
3.162 |
83.45 |
1.921 |
16.550 |
1.219 |
|
11 |
11 |
3.316 |
87.47 |
1.942 |
12.530 |
1.098 |
|
12 |
12 |
3.464 |
87.89 |
1.957 |
12.112 |
1.075 |
Table 4.17 Dissolution data of formulation A1
|
Sr. No. |
Time(h) |
Root t |
Cumu. % release |
Log Cumu. % release |
Cumu. % retained |
Log Cumu. % retained |
|
1 |
1 |
1.000 |
3.006 |
0.478 |
96.994 |
1.987 |
|
2 |
2 |
1.414 |
10.008 |
1.000 |
89.992 |
1.954 |
|
3 |
3 |
1.732 |
16.736 |
1.223 |
83.264 |
1.920 |
|
4 |
4 |
2.000 |
21.824 |
1.339 |
78.176 |
1.893 |
|
5 |
5 |
2.236 |
26.646 |
1.426 |
73.354 |
1.865 |
|
6 |
6 |
2.449 |
30.264 |
1.481 |
69.736 |
1.843 |
|
7 |
7 |
2.645 |
35.664 |
1.552 |
64.336 |
1.808 |
|
8 |
8 |
2.828 |
41.842 |
1.622 |
58.158 |
1.765 |
|
9 |
9 |
3.000 |
47.366 |
1.675 |
52.634 |
1.721 |
|
10 |
10 |
3.162 |
54.224 |
1.734 |
45.776 |
1.661 |
|
11 |
11 |
3.316 |
59.424 |
1.774 |
40.576 |
1.608 |
|
12 |
12 |
3.464 |
63.922 |
1.806 |
36.078 |
1.557 |
Table 4.18 Dissolution data of formulation A2
|
Sr. No. |
Time(h) |
Root t |
Cumu. % release |
Log Cumu. % release |
Cumu. % retained |
Log Cumu. % retained |
|
1 |
1 |
1.000 |
4.184 |
0.622 |
95.816 |
1.981 |
|
2 |
2 |
1.414 |
12.906 |
1.111 |
87.094 |
1.940 |
|
3 |
3 |
1.732 |
16.072 |
1.206 |
83.928 |
1.924 |
|
4 |
4 |
2.000 |
19.656 |
1.293 |
80.344 |
1.905 |
|
5 |
5 |
2.236 |
26.738 |
1.427 |
73.262 |
1.865 |
|
6 |
6 |
2.449 |
32.821 |
1.516 |
67.179 |
1.827 |
|
7 |
7 |
2.645 |
37.944 |
1.579 |
62.056 |
1.793 |
|
8 |
8 |
2.828 |
43.218 |
1.636 |
56.782 |
1.754 |
|
9 |
9 |
3.000 |
47.662 |
1.678 |
52.338 |
1.719 |
|
10 |
10 |
3.162 |
56.818 |
1.754 |
43.182 |
1.635 |
|
11 |
11 |
3.316 |
62.548 |
1.796 |
37.452 |
1.573 |
|
12 |
12 |
3.464 |
67.128 |
1.827 |
32.872 |
1.517 |
Table 4.19 Dissolution data of formulation A3
|
Sr. No. |
Time(h) |
Root t |
Cumu. % release |
Log Cumu. % release |
Cumu. % retained |
Log Cumu. % retained |
|
1 |
1 |
1.000 |
3.222 |
0.508 |
96.778 |
1.986 |
|
2 |
2 |
1.414 |
12.88 |
1.110 |
87.12 |
1.940 |
|
3 |
3 |
1.732 |
15.226 |
1.182 |
84.774 |
1.928 |
|
4 |
4 |
2.000 |
19.98 |
1.301 |
80.02 |
1.903 |
|
5 |
5 |
2.236 |
28.95 |
1.462 |
71.05 |
1.852 |
|
6 |
6 |
2.449 |
31.284 |
1.495 |
68.716 |
1.837 |
|
7 |
7 |
2.645 |
34.56 |
1.539 |
65.44 |
1.816 |
|
8 |
8 |
2.828 |
40.986 |
1.613 |
59.014 |
1.771 |
|
9 |
9 |
3.000 |
48.632 |
1.687 |
51.368 |
1.711 |
|
10 |
10 |
3.162 |
56.016 |
1.748 |
43.984 |
1.643 |
|
11 |
11 |
3.316 |
63.525 |
1.803 |
36.475 |
1.562 |
|
12 |
12 |
3.464 |
69.962 |
1.845 |
30.038 |
1.478 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4.3.2.8.2 In vivo floatability study 36, 37
Healthy beagle dog weighing approximately 15 kg was used to assess in vivo floating behavior. Ethical clearance for the handling of experimental animals was obtained from the institutional animal ethical committee (IAEC) constituted for the purpose. The animal was fasted for 12 h and the first X-ray photographed to ensure absence of radio opaque material in the stomach. The dog was made to swallow barium sulphate loaded microspheres of promising batch with 100ml of water after a light meal. During the experiment dog was not allowed to eat but water was provided ad libitum. At predetermined time intervals the radiograph of the abdomen was taken using an X-ray machine (Medford).
Figure 4.5In vivo floating ability
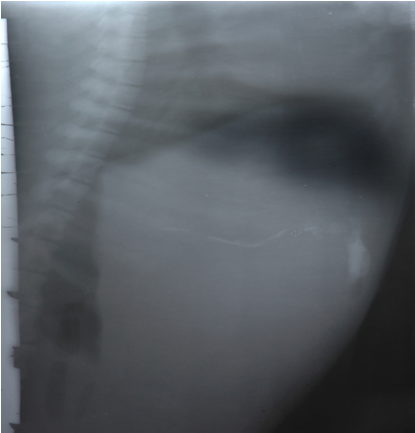
(Radiographic image) X-ray photograph of dog stomach showing floating behavior of MethocelK4M floating microspheres after 4 hr
4.3.2.8.3 Surface Topography (SEM) 29, 38
The surface morphology, shape and to confirm the hollow nature, microspheres were analyzed by scanning electron microscopy for selected batches (Leo, VP-435, Cambridge, UK). Photomicrographs were observed at required magnification operated with an acceleration voltage of 15 kV and working distance of 19 mm was maintained. Microspheres were mounted on the standard specimen-mounting stubs and were coated with a thin layer (20 nm) of gold by a sputter-coater unit to make the surface conductive. (VG Microtech, Uckfield, UK).
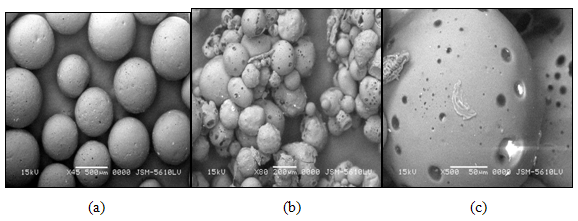
Figure 4.3 SEM of the kollidone SR microspheres (a), Acrycoat S100 microspheres (b) and cellulose acetate microspheres (c).
4.3.2.8.4 Kinetic modeling of drug dissolution profiles
The dissolution profile of all the batches was fitted to Zero order, First order39, 40 Higuchi41-43 and Korsemeyer and Peppas44-46 models to ascertain the kinetic modeling of drug release .The method of Bamba et al. was adopted for deciding the most appropriate model.47
Zero-Order
In many of the modified release dosage forms, particularly controlled or sustained release dosage forms (those dosage forms that release the drug in planned, predictable and slower than normal manner), is zero-order kinetics.
m = k * t
Where, k is zero order constant, m is % drug unreleased and t is the time. The plot of % drug unreleased (released) versus time is linear.
First-Order
Most conventional dosage form exhibits this dissolution mechanism. Some modified release preparations, particularly, prolonged release formulations, adhere to this type of dissolution pattern.
M=ea * e -bt
Where, a is the intercept and b is the slope.
It assumes that the drug molecules diffuse out through a gel like layer formed around the drug during the dissolution process. A plot of log % drug released versus time is linear.
Higuchi Model
A large number of modified release dosage form contain some sort of matrix system. In such instances, the drug dissolves form this matrix. The dissolution pattern of the drug is dictated by water penetration rate (diffusion controlled) and thus the following relationship applies:
m = 100 - q * square root of time
Where q is the Higuchi constant (% per square root of time) In Higuchi model, a plot of % drug unreleased (released) versus square root or time is linear.
Korsemeyer and Peppas Model
Mt /Mα =K t n
Where, n is diffusional exponent, If n is equal to one the release is zero order, if n is equal to 0.5 the release is best explained by Fickian diffusion, and if 0.5 < n < 1 then the release is through anomalous diffusion, n = 1 the release is best explained by case II transport i.e. zero-order release, and n > 1 (super case II transport). In this model, a plot of log % drug released versus log time is linear.
Figure 4.10 Firstorder kinetic plot of different formulations
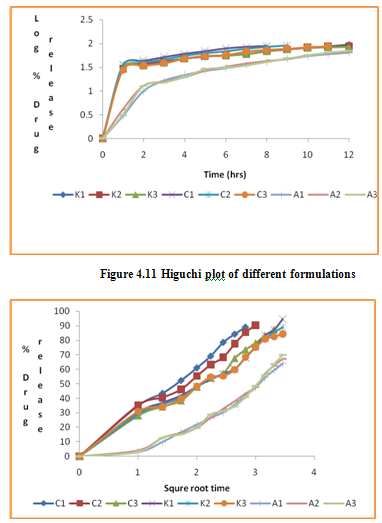
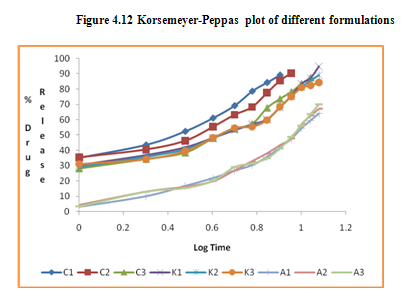
Table 4.20 Kinetic data of drug release from various formulations
|
Code |
Zero order |
First order |
Higuchi’s kinetics |
Peppas double log plots |
||||
|
Rate Constant (K) mg. min-1 |
Regression coefficient (R2) |
Rate Constant (K) mg. min-1 |
Regression Coefficient (R2) |
Rate constant (K) mg. min-1 |
Regression coefficient ( R2) |
Slope (n) |
Regression coefficient ( R2) |
|
|
C1 |
6.512 |
0.9438 |
-0.197 |
0.8541 |
25.582 |
0.9753 |
0.5614 |
0.9543 |
|
C2 |
6.3072 |
0.9401 |
-0.164 |
0.9399 |
24.949 |
0.9852 |
0.5673 |
0.9695 |
|
C3 |
6.051 |
0.9257 |
-0.143 |
0.9647 |
24.109 |
0.9842 |
0.5475 |
0.9542 |
|
K1 |
5.3592 |
0.9468 |
-0.102 |
0.9518 |
21.13 |
0.9858 |
0.5896 |
0.9790 |
|
K2 |
5.0046 |
0.9240 |
-0.092 |
0.9223 |
19.848 |
0.9734 |
0.5276 |
0.9527 |
|
K3 |
5.9786 |
0.9310 |
-0.136 |
0.9691 |
23.779 |
0.9863 |
0.5644 |
0.9693 |
|
A1 |
5.4035 |
0.9977 |
-0.087 |
0.9779 |
19.995 |
0.915 |
1.1545 |
0.982 |
|
A2 |
5.6227 |
0.9962 |
-0.094 |
0.9657 |
20.734 |
0.9073 |
1.0592 |
0.9873 |
|
A3 |
5.7235 |
0.9899 |
-0.098 |
0.9416 |
20.988 |
0.8916 |
1.1317 |
0.9741 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4.3.2.8.5 Stability study of the optimized batches
With the recent trend towards globalization of manufacturing operation, it is imperative that the final product be sufficiently rugged for marketing world wide under various climatic conditions including tropical, sub tropical and temperate. Stability studies were carried out as per ICH guidelines.48 The floating microspheres were placed in a screw capped glass containers and stored at room temperature, (25 ± 2°C), Humidity chamber (40°C, 75 % RH), and in Refrigerator (2-8°C) for a period of 90 days. The samples were assayed for drug content at regular intervals. The graph of percent drug content versus time (in days) was plotted.
Figure 4.13 Graphical representation of stability studies of prepared floating microspheres (Formulation Code K1, K2, K3)
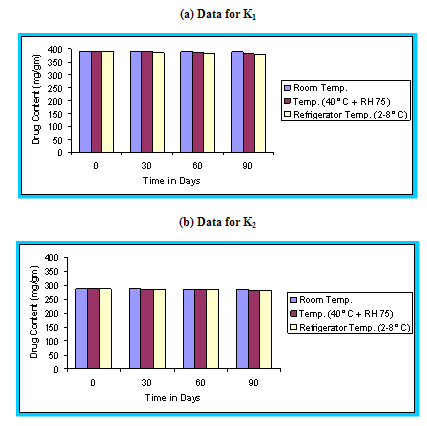
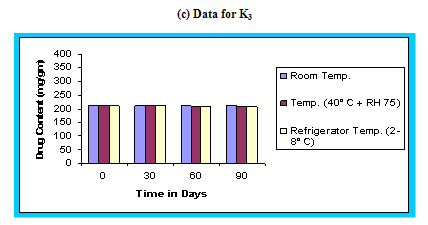
Figure 4.14 Graphical representation of stability studies of prepared floating microspheres (Formulation Code A1, A2, A3)
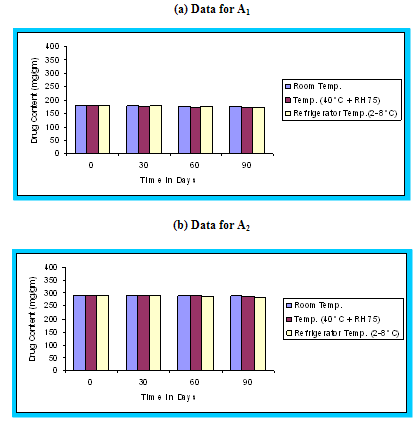
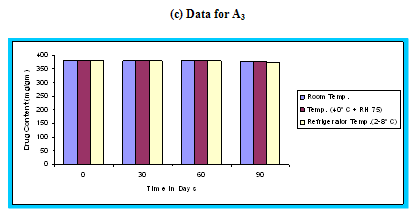
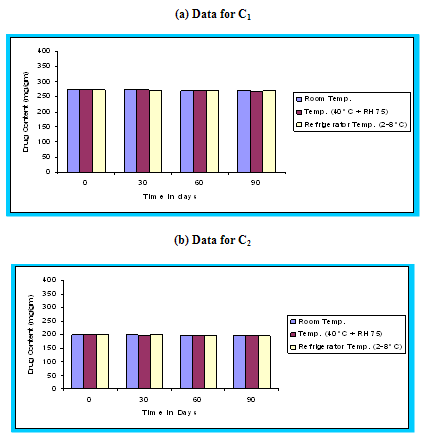
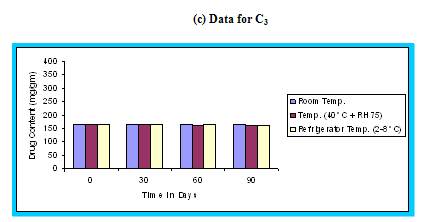
Figure 4.15 Graphical representation of stability studies of prepared floating microspheres (Formulation Code C1, C2, C3)
Result and Discussion:
Several Preformulation trials were undertaken for various proportions of drug and polymer by variation of the ethyl acetate-acetone ratio and dichloromethane-ethanol ratio. Kollidone SR, Acrycoat S 100 and Cellulose acetate were selected as matrixing agent considering its widespread applicability and excellent gelling activity in sustain release formulations and also having the pH-independent and reproducible drug release profile. It was found that Kollidone SR microspheres show desirable high drug content, yield, floatation and adequate release characteristics and hence was suitable for development of a controlled release system. No drug polymer incompatibility was noted in their FTIR spectra. The surface morphology and internal texture of floating microspheres were determined by scanning electron microscopy (SEM). Presence of pores were detected on the microspheres surface which increased in number and size after dissolution, it shows that the drug leach out through these channels.
The prepared microspheres were evaluated for the micromeritics properties. The average of three readings was taken. The mean particle size, flow properties and standard deviation were calculated. The low standard deviation of the measured mean particle size, % Compressibility,Hausner RatioandAngle of Reposeof all the 9 formulations ensures the uniformity of the microspheres prepared by emulsion solvent evaporation method. The mean particle size was found to be in the range of 252.45 ±4.63 µm to 463.64 ±3.68 µm. The variation in mean particle size could be due to variation in drug-polymer ratio. The % Compressibility of all the microspheres was found to be in the range of 13.86 ±0.26 to 21.55 ±1.88. The Hausner Ratio of all the microspheres was found to be in the range of 1.17 ±0.041 to 1.29 ±0.041. The Angle of Repose of all the microspheres was found to be in the range of 22.63 ±0.60 to 30.48 ±0.68. For the all formulations, % drug entrapped was found to vary from 72.9 % to 84.7 % and it shows that the drug entrapment is higher in microspheres containing Kollidone SR and lower in microspheres containing cellulose acetate. For the all formulations, % yield was found to vary 44.93 % to 97.40 % and it shows that the yield is higher in microspheres containing Kollidone SR and lower in microspheres containing cellulose acetate.
All formulations floated for more than 8 hours on the simulated gastric fluid USP. But more than 60 % microspheres of Kollidone SR and Acrycoat S 100 were floated for 12 hours whether microspheres containing cellulose acetate did not show buoyancy up to 12 hours.
In the present study, in vitro release studies of the floating microspheres were carried out in 0.1 N hydrochloric acid at 37°C for a maximum period of 12 hours. At different time intervals, samples were withdrawn and cumulative % drug release was calculated. The percentage drug release of all the formulations is presented in Figure. Out of 9 formulations tried, the formulation K1 was found to be satisfactory; since it showed prolonged and complete release with 94.75 % at end of 12 h.
The in vitro release data of all formulations were also subjected to model fitting analysis to know the mechanism of drug release from the formulations by treating the data according to zero order, first order, Higuchi and Korsmeyer-Peppas equation. The results are shown in Table. It can be interpreted from the result that the release of drug from the microspheres followed zero order kinetics. Further, the higuchi plot revealed that the drug release from the microspheres obeyed diffusion mechanism. It can be concluded that the formulation of microspheres (K1) containing Hydrochlorothiazide and Kollidone SR (1:1) seems to be promising and further in vivo study must be carried out to check the efficacy of preparations. In vivo floating ability of microspheres was studied; X-ray photograph of dog stomach with barium sulphate containing floating microspheres is shown in figure. Stability studies for all formulations were performed for three months, at room temperature (25 ± 2ºC), at refrigeration temperature (2 to 8ºC), and at 40ºC / RH 75%. The floating microspheres were stored at various above mentioned temperatures. The prepared microspheres were subjected for drug content analysis after every one month interval. Histogram was plotted between drug content (mg/gm) and time (In days), stability profile of different formulations at various temperatures is shown in figure. The data depicts that the floating microspheres stored at room temperature, refrigeration temperature, were found to be comparatively stable and at 40ºC / RH 75 % there was less than 5% degradation at the end of three months.
CONCLUSION
Multi unit gastroretentive drug delivery system has additional advantage of absence of dose dumping as in single unit drug delivery. The present investigation described the influence of viscosity and drug: polymer ratio on Hydrochlorothiazide release. The release and drug entrapment efficiency of the microspheres were affected by the different grade of Methocel. It was found that the Kollidone SR had a dominant role in the drug release from microspheres rather than Acrycoat S 100 and Cellulose acetate. And it can be given in hard gelatin capsule form. Therefore, it may be concluded that drug loaded floating microspheres in combination with Kollidone SR are a suitable drug delivery system for Hydrochlorothiazide and may be used for effective treatment of several cardiovascular disorders.
REFERENCES
1. Goodman and Gillman’s The pharmacological basis of therapeutics. Eleventh edition, p. 832-834.
2. Yeole .P.G., Khan, S. and Patel, V.F., Floating drug delivery systems: Need and development, Indian. J. Pharm. Sci., 67(3), 2005, 265-272.
3. Arora, S., Ali, J., Ahuja, A., Khar, R. K. and Baboota, S., Floating drug delivery systems .A review, AAPS PharmaScitech. 06(03), 2005, Article 47.
4. Vyas, S. P. & Khar, R. K., “Targeted and Controlled Drug Delivery Novel Carrier System”, first edition, CBS Publishers and Distributors, New Delhi, 2002, pp. 417-54.
5. Soppimath, K. S., Kulkarni, A. R., Aminabhavi, T. M., Development of Hollow Microspheres as Floating Controlled- Release Systems for Cardio-vascular Drugs. Drug Dev. Ind. Pharm., 2001 July; 27(6): 507-15
6. Sato, Y., Kawashima, Y., Takeuchi, H., Yama moto, H., In-vitro Evaluation of Floating and Drug Releasing Behaviour of Hollow Microspheres (Microballons) Prepared by Emulsion Solvent Diffusion Method, Eur. J. Pharm. Biopharm., 2004, 57 (2): 235-243.
7. Kumaresh, S. S., Anand Rao, R. K., Drug dev. Ind. Pharm., 27(6), 2001, 507-517.
8. Jain, N.K., “Controlled Novel Drug Delivery”, first edition, CBS Publishers and Distributors, New Delhi, 2002, pp.236-55.
9. Whitehead, L., Fell, J. T. and Collett, J. H., Development of a Gastro Retentive Dosage Forms, Eur. J. Pharm. Sci., 1996, 4 (1): S-182.
10. Cargill, R.et al., Pharma. Res., 5(8), 1988, 533.
11. Vyas, S. P. and Khar, R. K. “Targeted and Controlled Drug Delivery Novel Carrier System”, first edition, CBS Publishers and Distributors, New Delhi, 2002, pp. 417-54.
12. Liebermann, H. A., Lachmann, L. and Kenig, J. L., “ The Theory and Practice of Industrial Pharmacy”, third edition, Varghese Publications,1988, pp. 336-440
13. Soppimath, K. S., Kulkarni, A. R., Aminabhavi, T. M., Development of hollow microspheres as floating controlled release systems for cardio-vascular drugs, Drug Dev. Ind. Pharm., 2001 July; 27(6): 507-15.
14. Vyas, S.P. & Khar, R. K., “Targeted and Controlled Drug Delivery Novel Carrier System”, first edition, CBS Publishers and Distributors, New Delhi, 2002, pp. 487-94.
15. Chawla, G., Gupta, P., Koradia, V. and Bansal, A. K., Gastroretention- A means to address regional variability in intestinal drug absorption, Pharm. Tech., July 2003, 27(7): 50-51.
16. Jose, G. R., Omidian, H., Shah, K., Progress in Gastroretentive Drug Delivery Systems, Pharm. Tech., 2003, 152-154.
17. Garg, S. and Sharma, S., Gastro retentive drug delivery systems, Pharm. Tech., 2003, 13(1):160.
18. Indian Pharmacopoeia, Vol-II, The Controller of Publication, New Delhi, 1985, 540-41.
19. United States Pharmacopoeia, 22nd Edition, United States Pharmacopoeial Convention, Inc., Rock Ville, MD, 1990, 1444.
20. Gennaro, A. R., “Remington: The Science and Practice of Pharmacy”, 1995, 19th Edition, Mack Publishing Company, Eastern, Pennysylvania, pp.963.
21. Hardman, J. G., Limbird, L. E. in: Goodman and Gillman’s, “The Pharmacological Basis of Therapeutics”, 2001, 10th Ed, Mac Grawhill, New York, pp.1791.
22. Reynolds, J. E. F., In: Martindale, The Extra Pharmacopoeia, 31st Edn. The Pharmaceutical Press, London, 1996, 961-963.
23. Clarckson, P., Skinner, J., Hughes, B., Olsen, C., and Brkic, L., Newborn Services Drug Protocol.
24. Drug Today, 2000, pp 56.
25. United States Pharmacopoeia, 22nd Edition, United States Pharmacopoeial Convention, Inc., Rock Ville, MD, 1990, 1444.
26. Soppimath KS, Kulkarni AR, Aminabhavi TM. Development of Hollow Microspheres as Floating Controlled Release Systems For Cardiovascular Drugs. Drug Dev. Ind. Pharm. 2001, 27(6): 507-15.
27. Kale R, Rao BS, Sharma S, Ramanmurthy KV. Preparation and Evaluation of Floatable Drug Delivery System of Ketorolac Tromethamine. Int. J. Pharm. Excp. 2001, July-Sept, 64-65.
28. Patel, A., Ray S. and Thakur, R. In vitro evaluation and optimization of floating drug delivery system of metformin hydrochloride. DARU, 2006, 14, 57-64.
29. Rawat, M et al., Influence of Selected Formulation Variables on the Preparation of Enzyme-entrapped Eudragit S100 Microspheres. AAPS pharmscitech, 2007, 8(4), E1-E9.
30. Martin A., Physical Pharmaceutics, fourth edition. Lea Febiger, Philadelphia, pp.431-432.
31. Srivastava, A. K. et al., Floating microspheres of cimetidine: Formulation, characterization and in vitro evaluation. Acta Pharm. 55 (2005) 277–285.
32. Jain, S. K. et al., Evaluation of porous carrier-based floating orlistat microspheres for gastric delivery. AAPS pharmscitech, 2006, 7(4), E1-E9.
33. Iannuccelli, V., Coppi, G., Camaroni, R., Air compartment multiple unit system for prolonged gastric residence. Part I, formulation study. Int. J. Pharm. 1998, 174: 47-54.
34. Lee, J. H., Park, T. G., Development of oral drug delivery system using floating microspheres. J. Microencapsul. 1999; 16(6): 715-729.
35. Kawashima, Y., Niwa, T., Takeuchi, H., Hino, T., preparation of multiple unit hollow microspheres with acrylic resin containing tranilast and their drug release characteristics and floating behavior. J. Control Rel. 1991; 16:279-290.
36. Elkheshen, S. A., Eldeen B, Alsuwayeh, S., AlkhaIed, A., In vitro and in vivo Evaluation of Floating Controlled Release Dosage Forms. Pharm. Ind., 2004; 66, 1364-1372.
37. Klausner, E. A., Lavy, V., Friedman, M., Hoffman, A., Expandable gastroretentive dosage forms. J of Control. Rel.2003, 90, 143–162.
38. Behera, B. C., Sahoo, S. K., Dhal, S. And Gupta, B. K., Characterization of glipizide-loaded polymethacrylate microspheres prepared by an emulsion solvent evaporation method. Tropical J. of Pharm.Res.2008; 7 (1): 879-885.
39. Wagner J G. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J.pharm.Sci.1969; 58(10):1253-1257.
40. Gibaldi M, Feldman S. Establishment of sink conditions in dissolution rate determinations-theoretical considerations and application to nondisintegrating dosage forms. J. Pharm. Sci.1967; 56(10):1238-1242.
41. Higuchi T. Rate of Release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci.1961; 50(10):874-875.
42. Higuchi T. Mechanism of Sustained –Action Medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963; 52(12):1145-1149.
43. Cobby J., Mayersohn M., Walker, G. C. Influence of shape factors on kinetics of drugs release from matrix tablets II: Experimental, J. Pharm. Sci. 1974;63(5):732-737.
44. Korsemeyer, R., Gurny R., Peppas, N. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm 1983; 15:25-35.
45. Peppas, N. A. Analysis of fickian and non-fickian drug release from polymers. Pharm. Acta Helv. 1985; 60:110-111.
46. Harland, R. S., Sangalli M. E., Colombo, P, Peppas N. A. Drug/polymer matrix swelling and dissolution .Pharm. Res. 1988; 5: 488-494.
47. Bamba, M. Puisieusx, F. Release mechanisms in gel forming sustained release preparations. Int. J. Pharm. 1979; 2: 307-315.
48. Carstensen, J. T., Drug stability: Principle and practices, Marcal Dekker, New York, USA, 2nd edition, 1995, 538-550.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









