ABOUT AUTHORS:
*1Miss. Nikhil Lopez, 2Dileep K.J.
1Final Year B.Pharm student
2Assistant Professor
College of Pharmaceutical Sciences,
Govt. Medical college, Thiruvananthapuram
*nikkylopez90@gmail.com
1. INTRODUCTION
For the last few years, there has been an enhanced demand for more patient friendly dosage forms. Therefore, the demand for developing new technologies has been increasing every year. Since the development cost of a new drug molecule is very high, efforts are now being made by pharmaceutical companies to focus on the development of new drug dosage forms for existing drugs with improved efficacy and safety together with reduced dosing frequency, and the production of more cost effective dosage forms Fast disintegrating tablets (FDTs) have received ever-increasing demand during the last decade, and the field has become a rapidly growing area in the pharmaceutical industry.1
Fast dissolving Tablets are disintegrating and/or dissolve rapidly in the saliva without the need for water. Some tablets are designed to dissolve in saliva remarkably fast, within a few seconds, and are true fast-dissolving tablets. Others contain agents to enhance the rate of tablet disintegration in the oral cavity, and are more appropriately termed fast-disintegrating tablets, as they may take up to a minute to completely disintegrate. Oral delivery is currently the gold standard in the pharmaceutical industry where it is regarded as the safest, most convenient and most economical method of drug delivery having the highest patient compliance Fast or mouth dissolving tablets have been formulated for pediatric, geriatric, and bedridden patients and for active patients who are busy and travelling and may not have access to water. Many elderly persons will have difficulties in taking conventional oral dosage forms (viz., solutions, suspensions, tablets, and capsules) because of hand tremors and dysphagia. Other groups that may experience problems using conventional oral dosage forms include the mentally ill, the disabled, and patients who are uncooperative, on reduced liquid-intake plans, or are nauseated.
REFERENCE ID: PHARMATUTOR-ART-1848
Fast dissolving tablets are also called as mouth-dissolving tablets, melt-in mouth tablets, Oro dispersible tablets, rapimelts, porous tablets, quick dissolving etc. They disintegrate instantaneously releasing the drug which dissolve or disperses in the saliva.According to European pharmacopoeia, the ODT should disperse/disintegrate in less than three minutes. The basic approach in development of FDT is the use of superdisintegrants like cross linked carboxymethylcellulose (croscarmellose), sodium starch glycolate (primogel, explotab), polyvinylpyrollidone (polyplasdone) etc, which provide instantaneous disintegration of tablet after putting on tongue, their by release the drug in saliva. Another method is maximizing pore structure of the tablets by freeze drying and vacuum-drying. The bioavailability of some drugs may be increased due to absorption of drug in oral cavity and also due to pre gastric absorption of saliva containing dispersed drugs that pass down into the stomach. 2
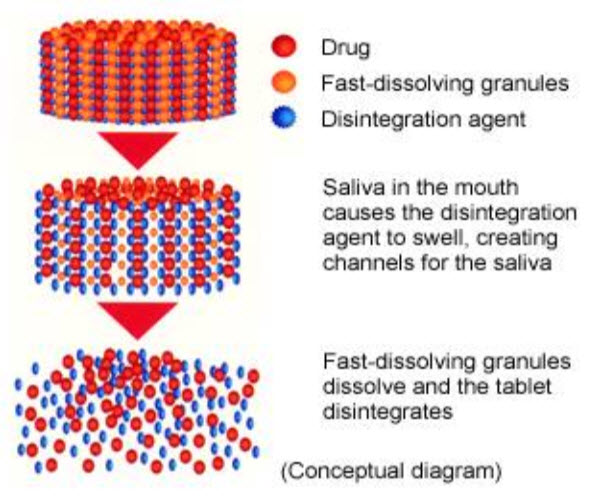
Figure 1. Conceptual diagram of FDt
BEnefits of fast dissolving tablets
- Administered without water, anywhere, any time.
- Suitability for geriatric and pediatric patients, who experience difficulties in swallowing and for the other groups that may experience problems using tablets due to being mentally ill, the developmentally disable and the patients who are un-cooperative, or are on reduced liquid intake plans or are nauseated.
- Beneficial in cases such as motion sickness, suede episodes of allergic attack or coughing, where an ultra rapid onset of action required.
- An increased bioavailability, particularly in cases of insoluble and hydrophobic drugs, due to rapid disintegration and dissolution of these tablets. Stability for longer duration of time, since the drug remains in solid dosage form till it is consumed. So, it combines advantage of solid dosage form in terms of stability and liquid dosage form in terms of bioavailability.3
2. DESIRABLE CHARACTERISTICS OF FAST DISSOLVING TABLETS4
Fast Disintegration
FDTs should disintegrate in the mouth without additional water or with a very small amount (e.g., 1–2 mL) of water. The disintegration fluid is provided by the saliva of the patient. The disintegrated tablet should become a soft paste or liquid suspension, which can provide good mouth feel and smooth swallowing. The “fast disintegration usually means disintegration of tablets in less than 1 minute, but it is preferred to have disintegration as soon as possible.
Taste of Active Ingredients
Because FDTs dissolve or disintegrate in the patient’s mouth, the drug will be partially dissolved in close proximity to the taste buds. After swallowing, there should be minimal or no residue in the mouth. A pleasant taste inside the mouth becomes critical for patient acceptance. Unless the drug is tasteless or does not have an undesirable taste, taste-masking techniques should be used.
An ideal taste-masking technology should provide drugs without grittiness and with good mouth feel. The amount of taste-masking materials used in the dosage forms should be kept low to avoid excessive increase in tablet size. The taste-masking technology should also be compatible with FDT formulations. For example, if drug particles are coated to minimize unpleasant taste, the coating should not be broken during compression or dissolved during wet granulation. Taste masking of bitter tasting drugs is critical to the success of the FDT formulations.
Drug Properties
For the ideal FDT technology, the drug properties should not significantly affect the tablet property. Many drug properties could potentially affect the performance of FDTs. For example, the solubility, crystal morphology, particle size, hygroscopicity, compressibility, and bulk density of a drug can significantly affect the final tablets’ characteristics, such as tablet strength and disintegration. The FDT technology should be versatile enough to accommodate unique properties of each drug.
tablet Strength and Porosity
Because FDTs are designed to have a quick dissolution/disintegration time, the tablet porosity is usually maximized to ensure fast water absorption into the tablets. The key properties of the tablets are fast absorption or wetting of water into the tablets and disintegration of associated particles into individual components for fast dissolution. This requires that excipients should have high wettability, and the tablet structure should also have a highly porous network5. Because the strength of a tablet is related to compression pressure, and porosity is inversely related to compression pressure, it is important to find the porosity that allows fast water absorption while maintaining high mechanical strength.
In addition, low compression pressure causes fast dissolving dosage forms to be soft, friable, and unsuitable for packaging in conventional blisters or bottles. A strategy to increase tablet mechanical strength without sacrificing tablet porosity or requiring a special packaging to handle fragile tablets should be provided.
Moisture Sensitivity
FDTs should have low sensitivity to humidity. This problem can be especially challenging because many highly water-soluble excipients are used in formulation to enhance fast dissolving properties as well as to create good mouth feel. Those highly water-soluble excipients are susceptible to moisture; some will even deliquesce at high humidity. A good package design or other strategy should be created to protect FDTs from various environmental conditions.
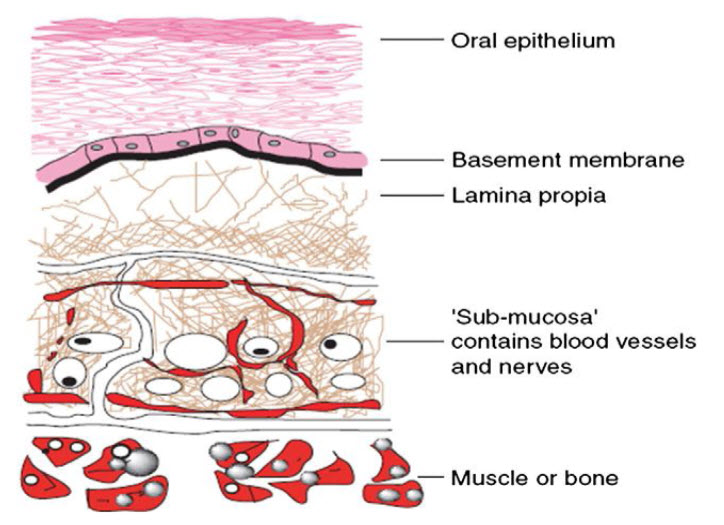
FIGURE2:SCHEMATIC DIAGRAM OF BUCCAL MUCOSA
3. FORMULATION ASPECTS OF FDTs
Important ingredients that are used in the formulation of FDTs should allow quick release of the drug, resulting in faster dissolution. This includes both the pharmacologically active ingredients (drug) and the excipients (additives).
3.1. SELECTION of drug candidate
Several factors may be considered while selecting an appropriate drug candidate for development of orally disintegrating tablets. The ultimate characteristics of a drugfor dissolution in mouth and pre gastric absorption from fastdissolving tablets include:
1. Free from bitter taste
2. Dose lower than 20mg
3. Small to moderate molecular weight
4. Good solubility in water and saliva
5. Partially unionized at salivary pH
6. Ability to diffuse and partition in to the epithelium of upper
GIT (log >1, or preferably>2)
7. Ability to permeate oral mucosal tissues.
There are no particular limitations as long as it is a substance which is used as a pharmaceutical active ingredient. Researchers have formulated ODT for various categories of drugs used for therapy in which rapid peak plasma concentration is required to achieve the desired pharmacological response.6 These include neuroleptics, cardiovascular agents, analgesics, anti allergic, anti-epileptics, anxiolytics, sedatives, hypnotics, diuretics, anti-parkinsonism agents, anti-bacterial agents and drugs used for erectile dysfunction. In contrast, the following characteristics may render unsuitable for delivery as an orally disintegrating tablet:
1. Short half life and frequent dosing.
2. Very bitter or otherwise unacceptable taste because taste masking cannot be successfully achieved.
3. Require controlled or sustained release7.
4. Combination with anticholinergics.
3.2. EXCIPIENTS
Mainly seen excipients in FDT are at least one disintegrate, a diluent, a lubricant and optionally swelling agent, a permeablizing agent, sweeteners and flavoring agents.
1. Superdisintegrants
Super disintegrate provide quick disintegration due to combined effect of swelling and water absorption by the formulation. Swelling index of the superdisintegrants is commonly studied in simulated saliva. Volume occupied by the material at the end of 4h should be noted and swelling index is calculated by the formula.
Swelling Index = [(Final volume – Initial volume)/initial volume)] X 100
Example: croscarmellose sodium, crospovidone, carmellose, carmellose calcium, sodium starch glycolate ion exchange resins (e.g. Indion 414) Sodium starch glycollate has good flowability than croscarmellose sodium. Cross povidone is fibrous nature and highly compactable.
Mechanism of Superdisintegrants8
There are four major mechanisms for tablets disintegration as follows:
1. Swelling:
Perhaps the most widely accepted general mechanism of action for tablet disintegration is swelling. Tablets with high porosity show poor disintegration due to lack of adequate swelling force. On the other hand, sufficient swelling force is exerted in the tablet with low porosity. It is worthwhile to note that if the packing fraction is very high, fluid is unable to penetrate in the tablet and disintegration is again slows down.
2. Porosity and capillary action (Wicking):
Disintegration by capillary action is always the first step. When we put the tablet into suitableaqueous medium, the medium penetrates into the tablet and replaces the air adsorbed on theparticles, which weakens the intermolecular bond and breaks the tablet into fine particles. Wateruptake by tablet depends upon hydrophilicity of the drug /excipient and on tableting conditions.For these types of disintegrants maintenance of porous structure and low interfacial tension towards aqueous fluid is necessary which helps in disintegration by creating a hydrophilic network around the drug particle
3. Due to disintegrating particle/particle repulsive forces
Another mechanism of disintegratn attempts to explain the swelling of tablet made with ‘nonswellable’disintegrants. Guyot-Hermann has proposed a particle repulsion theory based on the observation that nonswelling particle also cause disintegration of tablets. The electric repulsive forces between particles are the mechanism of disintegration and water is required for it.Researchers found that repulsion is secondary to wicking.
4. Due to deformation
During tablet compression, disintegranted particles get deformed and these deformed particles get into their normal structure when they come in contact with aqueous media or water.Occasionally, the swelling capacity of starch was improved when granules were extensively deformed during compression. This increase in size of the deformed particles produces a break up of the tablet. This may be a mechanism of starch and has only recently begun to be studied.
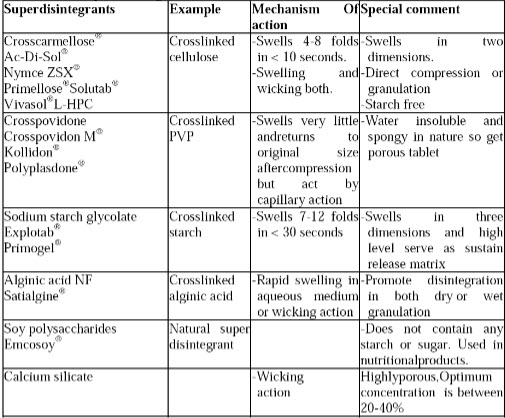
Table 1:list of super disintegrants
2. Binders Main role of Binders is to keep the composition of these fast melting tablets together during the compression stage. Binders can either be liquid,semi solid, solid or mixtures of varying molecular weights such as polyethylene glycol.Example: Binders commonly used are cellulosicpolymers, povidones, polyvinyl alcohols, and acrylic polymers. Acrylic polymers used are the ammoniomethacrylate copolymer, polyacrylate,and polymethacrylate.
3. Antistatic agent An antistatic agent is a compound used for treatment of materials or their surfaces in order to reduce or eliminate buildup of static electricity generally caused by the triboelectric effect.Example: colloidal silica (Aerosil), precipitated silica (Sylod.FP244), talc, maltodextrins, betacyclodextrin etc.
4. Lubricants Lubricants remove grittiness and assist in the drug transport mechanism from the mouth down into the stomach.Example: Magnesium stearate, stearic acid,leucine,sodium benzoate, talc, magnesium lauryl sulphate, liquid paraffin etc.
5. Flavours Example: Peppermint flavour, clove oil, anise oil, eucalyptus oil. Flavoring agents include, vanilla,citrus oils, fruit essences etc.
6. Sweeteners Example: Sorbitol, Mannitol, Maltitol solution, Maltitol, Xylitol, Erythritol, Sucrose, Fructose,Maltose, aspartame, sugars derivatives etc.
7. Fillers Example: Directly compressible spray dried Mannitol, Sorbitol, xylitol, calcium carbonate,magnesium carbonate, calcium phosphate,pregelatinized starch, magnesium trisilicate,aluminium hydroxide etc.
8. Surface active agents Example: sodium doecylsulfate,sodium laurylsulfate, Tweens, Spans,polyoxyethylene stearate.
4.FORMULATION TECHNOLOGIES
The techniques used to manufacture ODTs can be classified as:-
1) Conventional techniques
2) Patented techniques
Conventional Techniques
The various conventional technologies are developed for the preparation of Orally disintegrating drug delivery system that are Freeze drying9, Spray drying, Molding , Phase transition process, Melt granulation, Sublimation, Mass Extrusion, Cotton Candy Process, Direct compression method.
Patented Techniques
Rapid-dissolving characteristic of ODTs is generally attributed to fast penetration of water into tablet matrix resulting in its fast disintegration. Several technologies have been developed on the basis of formulation aspects and different processes and resulting dosage forms vary on several parameters like mechanical strength, porosity, dose, stability, taste, mouth feel, dissolution rate and overall bioavailability. Zydis,wow tab,Orasolv, Flashdose, Durasolv are some among them10.
4.1.Conventional Techniques
Freeze-Drying or Lyophilization
Freeze drying11 is the process in which water is sublimed from the product after it is frozen. This technique creates an amorphous porous structure that can dissolve rapidly. A typical procedure involved in the manufacturing of ODT using this technique is mentioned here. The active drug is dissolved or dispersed in an aqueous solution of a carrier/polymer. The mixture is done by weight and poured in the walls of the preformed blister packs. The trays holding the blister packs are passed through liquid nitrogen freezing tunnel to freeze the drug solution or dispersion. Then the frozen blister packs are placed in refrigerated cabinets to continue the freeze-drying.
After freeze-drying the aluminum foil backing is applied on a blister-sealing machine. Finally the blisters are packaged and shipped. Th entire freeze drying process is done at nonelevated temperatures to eliminate adverse thermal effects that may affect drug stability during processing.The freeze-drying technique has demonstrated improved absorption and increase in bioavailability12.
The major disadvantages of lyophillization technique are that it is expensive and time consuming; fragility makes conventional packaging unsuitable for these products and poor stability under stressed conditions
Sublimation
The key to rapid disintegration for mouth dissolving tablets is the presence of a porous structure in the tablet matrix. Conventional compressed tablets that contain highly water?soluble ingredients often fall to dissolve rapidly because of low porosity of the matrix. Hence, to generate porous matrix, volatile ingredients are used that are later subjected to a process of sublimation13. Inert solid ingredients that volatilize readily (e.g. urea, ammonium carbonate, ammonium bicarbonate, hexamethelene tetramine, camphor etc.) were added to the other tablet ingredients and the mixture is compressed into tablets. The volatile materials were then removed via sublimation, which generates porous structures. Additionally, several solvents (e.g. cyclohexane, benzene) can be also used as pore forming agents.
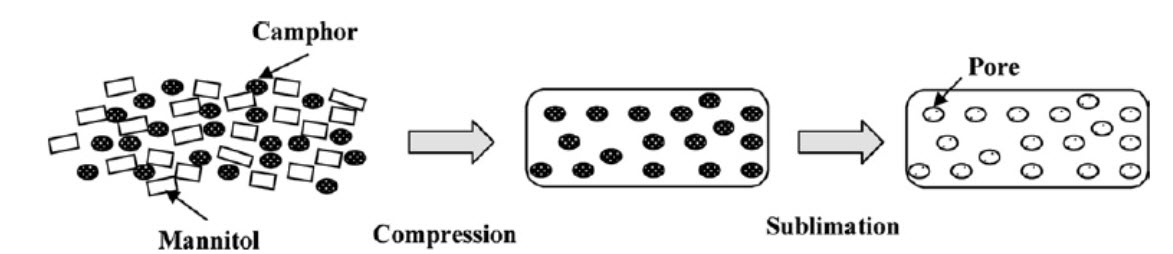
Figure 3:Schematic view of preparation of porous tablet using sublimation
DIRECT COMPACTION
Using a conventional tablet press to make fast-dissolving tablets is a very attractive method because of the low manufacturing cost and ease in technology transfer. However, the tablet press has been designed to make conventional tablets. When making conventional tablets, maintaining high tablet porosity is not a primary concern, and high compression force is used to ensure the tablet strength. Many strategies have been tried to achieve high porosity and adequate tablet strength using a tablet press. First, several granulation methods have been tried to obtain granules suitable for making FDTs. Wet granulation, dry granulation, spray drying, and flash heating methods have been tried. The second approach is to select special types of excipients as the main component for FDTs. The third approach is to compress tablets at low pressure and apply various after-treatments to the soft tablets. The three most widely used approaches are described in detail below.
1. Granulation Methods
a. Wet Granulation
It was found that even with effervescent agents presented in the tablet with lower than 5%, quick disintegration times could be achieved. Furthermore, it was also found that fast disintegration time could be achieved using only the acid component of the effervescent couple. In the patent, the formulation includes poly alcohols (e.g., mannitol, xylitol, sorbitol, maltitol, erythritol, and lactitol), 1–30% of an edible acid, and an active ingredient as the dry mixture. This mixture was wet granulated with an aqueous solution of a water-soluble or water-dispersible polymer (e.g., poly (ethylene glycols), carrageenan, and ethyl cellulose), which consisted of 1–10% of the final weight of the granule in a fluid bed.14 Granules with high porosity and low apparent density were obtained, and the tablets made by such granules had rapid disintegration times ranging from 3 to 30 seconds in the saliva.
First, nano particles were formed by mechanical grinding, precipitation, or any other suitable size reduction process. Those nano particles, less than 2 μm, were stabilized by surfactants. The particles were granulated with at least one pharmaceutically acceptable water-soluble or water-dispersible excipient using a fluid bed, and the granules were made into tablets. The tablets had complete disintegration or dissolution in less than 3 minutes.
b. Dry Granulation
Higher density alkali earth metal salts and water-soluble carbohydrates usually do not provide quick disintegration and a smooth mouth feel. Low-density alkali earth metal salts and water soluble carbohydrates are also difficult to compress and caused inadequate content uniformity. For these reasons, low-density alkali earth metal salts or water-soluble carbohydrates were pre compacted, and the resulting granules were compressed into tablets that could dissolve fast. In this process, a powdered material with a density of 0.2–0.55 g/m L was pre compacted to increase the density to 0.4–0.75 g/mL by applying a force ranging from 1 to 9 k N/cm. The resulting granules were compressed into tablets.
c. Melt Granulation
Abdelbary described a new approach of preparing FDTs with sufficient mechanical strength, involving the use of a hydrophilic waxy binder (Superpolystate, PEG-6-stearate) by melt granulation or wet granulation. Because Superpolystate is a waxy material with a melting point of 33–37 °C and a hydrophilic to lipid balance (HLB) value of 9, it will not only act as a binder and increase the physical strength of tablets but also help the disintegration of the tablets. In case of melt granulation, granules were prepared in a high-speed blade mixer at 40–44 °C, according to the conventional hot-melt procedure.15 For wet granulation, an oil-in-water emulsion of Superpolystate was used as the granulating agent. Then, granules were blended with croscarmellose, aspartame, and magnesium stearate and compressed into tablets. The melt granulation FDTs had better hardness results than the wet granulation FDTs. The disintegration times of melt granulation tablets, however, was more than 1 minute.
d. Spray Drying
This technology produces highly porous and fine powders as the processing solvent is evaporated during the process. Tablets manufactured from the spray-dried powder have been reported to disintegrate in less than 20 seconds in aqueous medium. The components included supporting agents composed of two polypeptide components of the same net charge (preferably non hydrolyzed and hydrolyzed gelatin), a bulking agent (mannitol) and a volatilizing agent. To maintain the net charges of the polypeptide components, an acidifying or alkalinizing agent was included. The mixtures of the above components were spray dried to obtain porous granules. The reason to usepolypeptide components of the same charge was that molecules would repel eachother even after spray drying, so porous and low-bulk-density particles could beformed. By incorporating a volatilizing agent (ethanol in most cases), the surfacetension of the droplets was further reduced during spray drying, and more poresand channels were created.16 The dissolution rate of the matrix was further increasedwhen combined with a bulking agent. A minimal amount of an effervescent agent was optionally included to further accelerate the dissolution rate. To aid in keepingthe tablets intact during handling, a thin coating of polymeric material could beapplied externally. This coating should not inhibit the capillary uptake of water duringdissolution.
Active ingredients can be microencapsulated or nano encapsulatedto further achieve taste masking.This formulation technique gives porous powder and disintegration time < 20 seconds.
e.Phase transition process
It is concluded that a combination of low and high melting point sugar alcohols, as well as a phase transition in the manufacturing process, are important for making FDTs without any special apparatus. FDT were produced by compressing powder containing erythritol (m. pt. 122°C), and xylitol (melting point: 93 95 °C), and then heating at about 93 °C for 15 min. After heating, the median pore size of the tablets was increased and tablet hardness was also increased. The increase of tablet hardness with heating and storage did not depend on the crystal state of the lower melting point alcohol.17
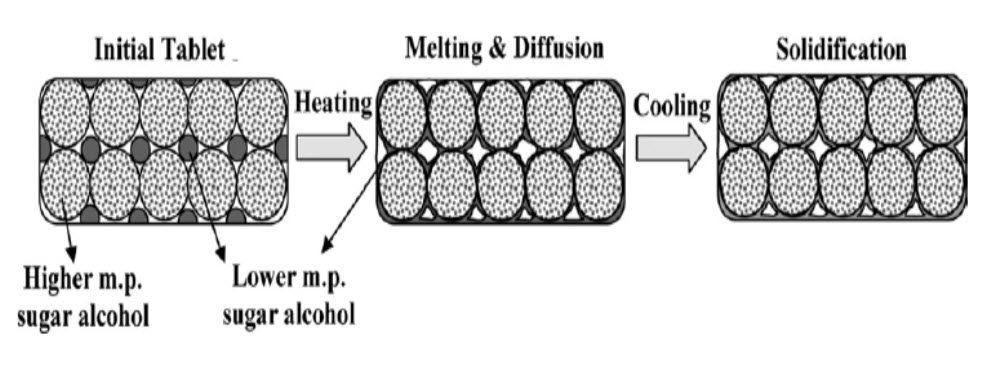
Figure 4:Schematic illustration of a fast disintegration tablet prepared by the phase transition method using a higher melting (erythritol) and a lower melting (xylitol) sugar alcohol.
f. Flash Heat Process
The Shearform Technology uses a unique spinning mechanism to produce a floss-like crystalline structure, much like cotton candy. In this process, the feedstock is subjected to centrifugal force and to a temperature gradient simultaneously. An internal flow is created by this condition to force the fl owing mass out of the opening provided in the perimeter of a spinning head. The mass is cooled down as it comes out of the opening to form a discrete fiber structure, as seen in cotton candy. The speed of spinning is about 3,000–4000 rpm, and the temperature gradient is about 180–250°C. The carrier materials include saccharides, polysaccharides, and mixtures thereof. There were two systems used to create the Shearform floss having self-binding properties.
The first system was named a single floss or unifloss. Typical flosses of this kind, made of sucrose, sorbitol, and xylitol, yielded effective self binding properties. The second system used two separate flosses. One was xylitol containing binder flosses and the other was base flosses that contain different sugar alcohols or saccharide. When the two flosses were combined, it was termed a dual floss system. The produced floss needed to be recrystallized to form freely fl owing granules with self-binding properties. Two techniques were used in recrystallization. One was using crystallization enhancers including ethanol, polyvinylpyrollidone18, water (e.g., moisture), glycerin, and radiant energy (e.g., microwaves). The other was using crystallization modifiers, which were included in floss ingredients at 0.01–20.0% the weight of the floss. Typical crystallization modifiers were surfactants having an HLB of about 6 or more. A hygroscopic material such as xylitol must be present in the system to provide good self-binding characteristics to the final matrices. In order to produce and control the self-binding properties, this hygroscopic material must have a substantially higher hygroscopicity than that of the carrier carbohydrate (e.g., sucrose). The initial floss coming out of the spinning machine is in its amorphous state. However, because of its tendency to pick up moisture in the floss and induce crystallization by crystallization enhancers or modifiers, the floss recrystallizes into a more crystalline structure. Because of intimate contacts among all components in the matrix, recrystallization of one component can have significant impact on the characteristics of surrounding components and further change the properties of the matrix as a whole. The floss gradually loses its amorphous character as the recrystallization continues. The flowability of the floss is enhanced to make it suitable for the conventional tableting process.
g. Cotton candy process
This process is so named as it utilizes a unique spinning mechanism to produce floss-like crystalline structure, which mimic cotton candy. Cotton candy process 10 involves formation of matrix of polysaccharides or saccharides by simultaneous action of flash melting and spinning. The matrix formed is partially re-crystallized to have improved flow properties and compressibility. This candy floss matrix is then milled and blended with active ingredients and excipients and subsequently compressed to MDTs.
h. Mass extrusion:
This technology involves softening the active blend using the solvent mixture of water-soluble polyethylene glycol and methanol and subsequent expulsion of soften mass through the extruder or syringe to get a cylinder of products into even segments using heated blade.
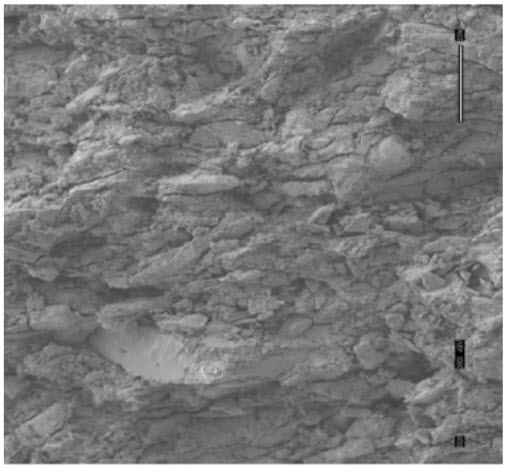
Figure5: Scanning electron microscope of FDT (clarithrinTM RediTabsTM)
4.2.patented technologies
A. zydis technology
The Zydis technology is the most well known example of the freeze drying method. In the Zydis formulations, the drug is physically trapped in a matrix composed of two components, a saccharide (e.g., mannitol) and a polymer.1?-12 The carrier polymers commonly used in the Zydis system include (partially hydrolyzed) gelatin, hydrolyzed dextran, dextrin, alginates, poly(vinyl alcohol), polyvinyl pyrrolidone, acacia, and the mixtures. After the solution or dispersion is filled into blister cavities, it is then frozen in a liquid nitrogen freezing tunnel. The solvent in the frozen state is removed to produce porous wafers. Because the units are fragile and light-weight, they cannot withstand the pressure of being pushed through the foil of a conventional blister. A special peelable backing foil is used to package the Zydis units19. Because the water content in the final freeze-dried product is too low for microbes to grow, the Zydis formulation is also self-preserving.
However, the Zydis formulation is so sensitive to moisture that the tablet may melt between sweaty fingers when it is taken out of the package. This dosage form may degrade at humidity greater than 65%, so a pinhole or minor damage to the package will lead to collapse of the tablet. There are some requirements for the Zydis technology. The drug should be chemically stable and water insoluble, with a particle size smaller than 50 μm. Precipitation may occur during manufacturing if large drug particles are used. The water soluble drugs may form eutectic mixtures that cannot be frozen adequately to form the rigid structure necessary to support itself after solvent is removed, which may cause collapse of the freeze dried cake. For this reason, the dose for water-soluble drugs is usually limited to 60 mg3. Higher drug dosing can be accommodated without losing the rapid disintegration property, however, if a thickened (i.e., paste-like) form of an oil-in-water emulsion is directly placed in the blister cell.
B.orasolv and durasolv technology
OraSolv technology (Cima Labs) produces tablets by low compression pressure. It uses an effervescent disintegration pair that releases gas upon contact with water. The widely used effervescent disintegration pairs usually include an acid source and a carbonate source. The acid sources include citric acid, tartaric acid, malic acid, fumaric acid, adipic acid, and succinic acids. The carbonate sources include sodium bicarbonate, sodium carbonate, potassium bicarbonate, and potassium carbonate.
The carbon dioxide evolved from the reaction may provide some “fizzing” sensation, which is a positive organoleptic sensation. The amount of effervescent agent is in general about 20–25% of the total weight of the tablet.
Because of the soft and fragile nature of OraSolv tablets, a special packaging system, known as PakSolv, was developed to protect the tablets from breaking during transport and storage. PakSolv is a “dome-shaped” blister package that prevents the vertical movement of the tablet within the depressions, because the diameter of the lower portion of the dome is too narrow to accommodate the tablet. PakSolv also offers light, moisture, and child resistance. As a second-generation technology, the DuraSolv technology was developed by Ciba to provide stronger tablets for packaging in blisters or bottles (Amborn and Tiger.
The key ingredients in this formulation are non-direct compression filler and lubricant. The particle size of the non-direct compression filler is preferably between about 20 and 65 μm, while for direct compressible fillers at least 85% of the particles are over 100 μm in size. These non-direct compression fillers, such as dextrose, mannitol, sorbitol, lactose, and sucrose, have the advantage of quick dissolution and avoid some of the gritty or sandy texture usually present in direct compressible versions of the sugar. The amount of non direct compression filler is usually about 60–95% of the total tablet weight. The tablets have low friability, which is about 2% or less when tested according to the USP, and the hardness of the tablets is at least about 15–20 N. The disintegration time is less than 60 seconds. It is interesting to note that in comparison with the conventional tablet formulations, higher amounts of hydrophobic lubricants, such as magnesium stearate, can be added to the formulation with non-direct compression fillers as the main component. About 1–2.5% of lubricant can be added to the formulation, compared with 0.2–1% of lubricant in conventional tablets. The lubricant blending time scan also be increased to 10–25 minutes or longer. Relatively modest compressive force is needed to compress the formulation. This method can produce tablets by the direct compression method and use conventional tableting methodologies and conventional package equipment. Thus, the production cost is significantly decreased.
C.Flashtab Technology
Flashtab technology produces tablets by compression of granular excipients. This technology uses almost the same excipients as do conventional compressed tablets. Excipients used in this technology comprise two groups of components: disintegrating agents, such as carboxymethylcellulose or insoluble reticulated polyvinyl pyrrolidone; and swelling agents, such as carboxymethylcellulose, starch, modified starch, carboxymethylated starch, microcrystalline cellulose, and possibly directly compressible sugars. The mixture of excipients is prepared by either dry or wet granulation methods. The produced tablets are known to have satisfactory physical resistance and disintegrate in the mouth within 1 minute.
D.AdvaTab Technology
AdvaTab technology produces FDT tablets based on a proprietary tablet composition that was designed and patented by Kyowa Hakko Kogyo; in which the lubrication is dispensed onto each tablet by using a spray during the production process. Traditional tablets are produced using an internal lubrication system, which disperses lubricant on the inside and the surface of the tablets. This method can decrease tablet mechanical strength. AdvaTab is produced using 10–30 times less hydrophobic lubricant and can be 30–0% stronger than conventional tablets. As a result, the tablets are hard and durable yet do not impede liquid entry upon contact with saliva. AdvaTab can handle high drug loading and coated drug particles. Importantly, the technology does not require specialty packaging and, as a result, can be packaged in both standard bottles and push-through blisters
E.Dispersible Tablet Technology
Dihydroergotoxine is poorly soluble in water in the free base form. An improved dissolution rate of dihydroergotoxine methane sulphonate was observed with dispersible tablets containing 0.8– 10%, preferably about 4% by weight, of an organic acid. One of the essential excipients in the cimetidine formulation was a disintegrating agent. It provides rapid swelling and/or good wetting capability to the tablets and thereby a quick disintegration .The disintegrating agents include starch or modified starches, microcrystalline cellulose, alginic acid, cross-linked sodium carboxy methyl cellulose, and cyclodextrin polymers. A combination of two or more disintegrating agents produced better disintegration results.
F.WOWTAB Technology
WOWTAB technology employs a combination of low and high-moldability saccharides to produce fast-dissolving tablets using conventional granulation and tableting techniques According to the patent, saccharides were divided into two groups: those with high moldability and those with low moldability. Low moldability saccharides produce tablets with hardness between 0 and 2 kg, when 150 mg of such a saccharide is compressed under pressure of 10–50 kg/cm2 using a die 8 mm in diameter. The typical low moldability saccharides include lactose, mannitol, glucose, sucrose, and xylitol. High-moldability saccharides produce tablets with hardness above 2 kg when prepared under the identical conditions. The typical high- moldability saccharides are maltose, maltitol, sorbitol, and oligosaccharides. When tablets are made by compressing a saccharide having low moldability or high moldability alone, the desired properties of adequate hardness and quick disintegration in the mouth cannot be achieved simultaneously. Moreover, if saccharides having low moldability and high moldability are mixed (physical mixture) before tableting, quick disintegration and dissolution in the mouth cannot be obtained. For this reason, a saccharide having low moldability was granulated with a saccharide having high moldability as a binder. The low-moldability saccharides were used as the main component. The tablets show an adequate hardness and fast disintegration and dissolution when put in the mouth20
G.FROSTA TECHNOLOGY
The Frosta technology combines the major advantages of fast disintegration of freeze-dried formulation and the high mechanical strength of compressed tablets. Moreover, the technology utilises a conventional wet granulation process and tablet press for cost-effective production of the tablets with desirable properties. The Frosta technology is based on compression of highly plastic granules at low pressures to prepare FDTs. The highly plastic granules are composed of three components: a plastic material, a water-penetration-enhancer and a wet binder. A plastic material and a water-penetration-enhancer can be the same material. Each of the three components plays an important role in obtaining tablets with high strength and fast disintegration time. The key benefits of the Frosta technology are:
• Fast disintegration in the mouth – between 5 and 50 seconds, depending on the tablet size.
• Low manufacturing cost – the same as making conventional tablets.
• One-step granulation processing.
• Strong mechanical property with friability less than 1%; and
• Multi tabletpackaging, with dozens of tablets in one bottle.
Any granulation method can be used to prepare highly plastic granules. Tablet excipients with porous structure can have more plastic deformation than non-porous counterparts when compressed under the same conditions. Moreover, when the same material is used for making FDTs, the mechanical strength of tablets is increased when porous granules are used.
The Frosta tablets are mechanically strong with friability less than 1%, and are relatively stable in an open-air environment. They are robust enough to be packaged in multi tablet vials. When tested in vitro, Frosta tablets absorbed water very quickly and disintegrated instantly upon contact with water, forming a paste for easy swallowing. Depending on the size, the Frosta tablets can disintegrate in less than 10 seconds after placing them in the oral cavity for easy swallowing. Scanning electron microscopy of the inner structure of a Frosta tablet showed the presence of a lot of pores between the granules throughout the table matrix. It is these pores that increase the absorption of water by capillary force. Upon contact with the saliva orwater, the granules can be easily dissociated, and the whole tablet disintegrates to form a paste, which is very easy to swallow.The key properties of the Frosta tablet are its highly porous structure, offering fast disintegration in the mouth, and its mechanical strength, due to the highly plastic granules.
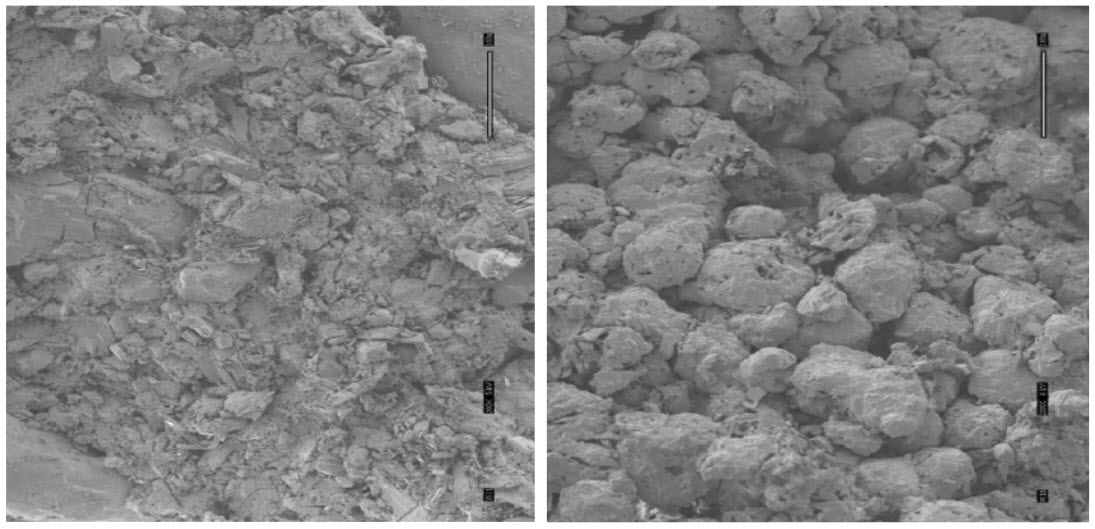
Figure 6(A) shows an inner structure of a BenadrylRFastmelt™, which used WOWTAB technologyFigure 6(B) shows an inner structure of a Frosta tablet containing vitamin B12 and folic acid. In this approach, highly plastic granules are compressed to form a tablet. As shown in the figure, there are empty spaces between granules where water can be absorbed by capillary force. The granules are then easily dissociated, and the whole tablet melts to form a paste that is easy to swallow.
H.Nanocrystal technology
For fast dissolving tablets, Élan’s proprietary Nanocrystal technology can enable formulation and improve compound activity and final product characteristics. Decreasing particle size increases the surface area, which leads to an increase in dissolution rate. This can be accomplished predictably and efficiently using Nanocrystal technology. Nanocrystal particles are small particles of drug substance, typically less than 1000 nanometers (nm) in diameter, which are produced by milling the drug substance using a proprietary wet milling technique.
Nano Crystal Fast dissolving technology provides for:
• Pharmacokinetic benefits of orally administered nano particles (<2 microns) in the form of a rapidly disintegrating tablet matrix.
• Product differentiation based upon a combination of proprietary and patent?protected technology elements.
• Cost?effective manufacturing processes that utilize conventional, scalable unit operations.
• Exceptional durability, enabling use of conventional packaging equipment and formats (i.e., bottles and/or blisters).
• Wide range of doses (up to 200mg of API per unit).
• Use of conventional, compendial inactive components.
• Employment of non?moisture sensitive in-actives.
Nanocrystal colloidal dispersions of drug substance are combined with water?soluble GRAS (Generally Regarded as Safe) ingredients, filled into blisters, and lyophilized. The resultant wafers are remarkably robust, yet dissolve in very small quantities of water in seconds. This approach is especially attractive when working with highly potent or hazardous materials because it avoids manufacturing operations (e.g., granulation, blending, and tableting) that generate large quantities of aerosolized powder and present much higher risk of exposure. The freeze?drying approach also enables small quantities of drug to be converted into FDT dosage forms because manufacturing losses are negligible.
I.Pharmaburst Technology
Pharmaburst technology (SPI Pharma, New Castle, Delaware) uses off -the-shelf co processed excipients to create an FDT that, depending on the type of active and loading (up to 700 mg), dissolves within 30–40 seconds. The quantity of Pharmaburst™ required in a formulation depends on the active in the tablet. It is necessary to carry out initial studies on a formulation by varying the amount of Pharmaburst™ from 50 to 80%, depending on the desired mouth feel and disintegration time. The process involves a dry blend of a drug, flavor, and lubricant that are compressed into tablets on a standard tablet press with stock tooling. The manufacture process can be carried out under normal temperature and humanity conditions. The tablets can be packaged in blister packs or bottle.
J.OraQuick technology
The OraQuick fast?dissolving/disintegrating tablet formulation utilizes a patented taste masking technology. KV Pharmaceutical claims its microsphere technology, known as Micro Mask, has superior mouth feel over taste?masking alternatives. The taste masking process does not utilize solvents of any kind, and therefore leads to faster and more efficient production. Also, lower heat of production than alternative fast?dissolving/disintegrating technologies makes OraQuick appropriate for heat?sensitive drugs. KV Pharmaceutical also claims that the matrix that surrounds and protects the drug powder in microencapsulated particles is more pliable, meaning tablets can be compressed to achieve significant mechanical strength without disrupting taste masking. OraQuick claims quick dissolution in a matter of seconds, with good taste masking. There are no products using the OraQuick technology currently on the market, but KV Pharmaceutical has products in development such as analgesics, scheduled drugs, cough and cold, psychotropics, and anti?infectives
K.Quick –Dis technology
Lavipharm Laboratories Inc. (Lavipharm) has invented an ideal intraoral fast?dissolving drug delivery system, which satisfies the unmet needs of the market. The novel intraoral drug delivery system, trademarked Quick?Dis™, is Lavipharm’s proprietary patented technology and is a thin, flexible, and quick?dissolving film. The film is placed on the top or the floor of the tongue. It is retained at the site of application and rapidly releases the active agent for local and/or systemic absorption. The Quick?Dis™ drug delivery system can be provided in various packaging configurations, ranging from unit?dose pouches to multiple?dose blister packages. The typical disintegration time, which is defined as the time at which the film begins to break when brought into contact with water, is only 5 to 10 seconds for the Quick?Dis™ film with a thickness of 2 mm. The dissolving time, which is defined as the time at which not less than 80% of the tested film is dissolved in aqueous media, is around 30 seconds for Quick Dis™ film with a thickness of 2 mm. The typical release profile of an active ingredient exhibited by a Quick?Dis™ drug delivery system is 50% released within 30 seconds and 95% within 1 minute.21
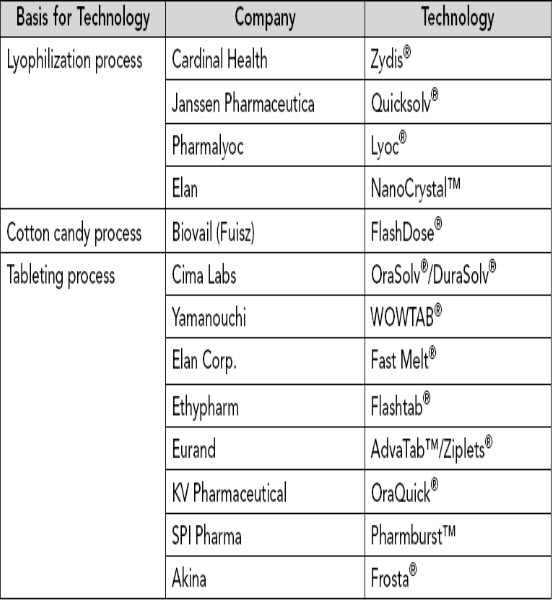
TABLE2. Examples of Commercially Available, Preapproval, or Submitted Orally Disintegrating Tablet Products
5. evaluation of fast dissolving tablets
Evaluation parameters of tablets mentioned in the Pharmacopoeias need to be assessed, along with some special tests. The quality of tablet, once formulated by rule, is generally dictated by the quality of physicochemical properties of blends .There are many formulation and process variables involved in mixing and all these can affect the characteristics of blends produced.
5.1.EVALUATION of blends before compression: preformulation studies
The various characteristics of blends to be tested before compression are
1. Angle of repose:
Angle of repose is determined by using funnel method. The accurately weighed blend is taken in a funnel. The height of the funnel is adjusted in such a way that the tip of the funnel just touches the apex of the heap of blend. The drug (as solid dispersion)-excipient blend is allow to flow through the funnel freely on to the surface. The diameter of the powder cone is measured and angle of repose is calculated using the following equation.
Tan ? = h/r (Where h and r are the height of cone and radius cone base respectively)
Table 3:angle of repose as an indication of powder properties.
Bulk density:
Apparent bulk density is determined by pouring a weighed quantity of blend into graduated cylinder and measuring the volume and weight. Bulk density can be calculated by using following formula:
Bulk density = Weight of the powder / Volume of the packing.
Tapped density:
It is determined by placing a graduated cylinder, containing a known mass of drug-excipients blend. The cylinder is allowed to fall under its own weight onto a hard surface from the height of 10 cm at 2 second intervals. The tapping is continued until no further change in volume is noted. Tapped density can be calculated by using following formula:
Tapped Density = (Weight of the powder / volume of the tapped packing)
Compressibility index:
The Compressibility Index of the blends is determined by compressibility index. Compressibility Index can be calculated by using following formul
DETERMINATION OF DISINTEGRATION TIME OF FDTs
should be strong enough to survive rough handling during manufacturing and shipping processes, and yet friable enough to instantly dissolve or disintegrate into small particles for easy swallowing by the patient. Conventional disintegration tests for ordinary tablets may not allow precise measurement of the disintegration time of FDTs because of their fast disintegration. It is also hard to distinguish among FDTs, which release their ingredients very quickly. In vitro testing may not always reflect the real in vivo disintegration of tablets. In general, the method described in the US Pharmacopoeia can produce data for evaluation of the disintegration time; however, no additional information might be extracted. It is also possible to evaluate the tendency of the disintegration kinetics by visual examination. However, these evaluations are not sufficiently objective. When developing FDT formulations, it is important to evaluate the effect of different excipients on the disintegration time. In order to predict the disintegration time of FDTs and the effects of different formulation parameters, a few methods have been proposed. It is important to define a suitable method to better distinguish between the disintegration times of different FDTs and to find better correlation between in vitro and in vivo data. 24 To achieve this goal, a modified dissolution apparatus was applied to FDTs with disintegration times too fast to distinguish the differences between the tablets when the conventional methods were used.
1. Modified US Pharmacopoeia Method
Instead of using the disintegration apparatus described in the US Pharmacopoeia, a modified method has been proposed. The disintegration apparatus was the same as the USP dissolution test Apparatus 2, which uses a paddle stirring element and 1000-mL cylindrical vessel at 37 °C. Distilled water was chosen for the disintegration medium instead of a buffer solution. A tablet to be tested was put on the bottom of a sinker, which was placed in the middle of the vessel and hung by a hook to the lid of the vessel with a distance of 6–8.5 cm. Disintegration time was determined at the point at which the tablet disintegrated and passed through the screen of the sinker completely. The opening of mesh of the sinker was 3–3.5 mm in height and 3.5–4 mm in width.
2. Texture Analyzer Method
The Texture Analyzer (Stable Micro Systems, U.K.) was applied to measure the beginning and ending time of disintegration. A tablet was adhered to the bottom of a probe, which was attached to the load cell with a very thin layer of glue or double-sided tape. A small amount of water, usually 0.4 m L, in a beaker or petridish was used as a disintegration medium at room temperature. The tablet was submerged in water and compressed against the bottom of the beaker or petridish with a constant pressure. The beaker size could be varied, and the beaker could even be a water bath to keep the temperature constant. The instrument was programmed to apply a moderate force for up to 60 seconds so that the penetration distance could be measured as the tablet was compressed while submerged in the water. The probe distance would be steady as the tablet remained cohesive. However, as the tablet disintegrated, the compression distances increased, because the probe had to keep the pressure constant. The time for the tablet to disintegrate was determined by measuring the distance the probe traveled into the tablet. Typical time-distance profiles generated by the Texture Analyzer software enabled the calculation of beginning and ending of disintegration time.
3. CCD Camera Method 25
The CCD camera apparatus comprises two distinct sections—a disintegration component and a measurement device. The mode of measurement involves the continuous acquisition of pictures by the CCD camera to record the time course of disintegration. The acquired pictures 1 Fast Dissolving Tablets are simultaneously transferred to the computer and stored. The key point of this apparatus is to combine the detailed pictures obtained by the CCD camera. The disintegration apparatus consists of a plastic cell partitioned into two parts: one component comprises an inner tank containing a stirring bar, a grid fabricated from stainless-steel, and a disintegration medium (distilled water, 200 m L, 37± 2 °C); the second component is an outer tank of thermo stated water. The grid is constructed of three hollow areas equidistant from the center. These hollow points represent the position of the tablets, and a support is added for each tablet to avoid movement during the disintegration test. The CCD camera method permits documentation of the disintegration time course with sequentially obtained pictures. The computer enables calculation of the surface area of each tablet at any time point, as well as the design of graphs that show decrease in the tablet surface area as a function of time. The disintegration time and the area under the curve can be calculated from these graphs as qualitative parameters that can be correlated to the oral disintegration time. Consequently, results depend on the direction and focal length of the camera relative to the tablet. The disadvantage of the method involves difficulty associated with the application of mechanical stress to test tablets. Thus, the time required for a single test is several minutes, which is greater than that for the in vivo disintegration time. The other methods include rotary shaft method and sieve method.
IN VIVO DETERMINATION OF DISINTEGRATION TIME
In vivo disintegration tests of FDTs 26 can be conducted on volunteers who are usually randomized to receive the treatments and then directed to clean their mouths with water. Tablets are placed on their tongues, and the time for disintegration is measured by immediately starting a stopwatch. The volunteers are allowed to move FDTs against the upper roof of the mouth with their tongue and to cause a gentle tumbling action on the tablet without biting on it or tumbling it from side to side. Immediately after the last noticeable granule has disintegrated, the stopwatch is stopped and the time recorded. 1 Fast Dissolving Tablets
FUTURE POTENTIAL
Fast dissolving tablets establish an innovative dosage form which overcomes the difficulties of swallowing and geriatric populations The clinical studies show FDTs can improve patient compliance, provide a rapid onset of action, and increase bioavailability. Considering the many benefits of FDTs, it is only a matter of time until a majority of oral formulations are prepared in FDT forms. The basic method followed by all the presently available technologies involved in the formulation of mouth dissolving tablets is to maximize the porous structure of the tablet matrix and incorporate super disintegrating agents in optimum concentration so as to achieve rapid disintegration and instantaneous dissolution of the tablet along with good taste masking possessions and excellent mechanical strength. 27 The availability of the various technologies and manifold benefits of fast dissolving tablets will surely increase its popularity in the near future. These dosage forms may be suitable for the oral delivery of drugs such as protein and peptide-based therapeutics that have limited bioavailability when administered by conventional tablets. These products usually degrade rapidly in the stomach. Should next generation drugs are predominantly protein or peptide based, tablets may no longer be the dominant format for dosing such moieties. Injections generally are not favored for use by patients unless facilitated by sophisticated auto-injectors. The developments of enhanced oral protein delivery technology by FDTs which may release these drugs in the oral cavity are very promising for the delivery of high molecular weight protein and peptide 28 . The future of FDTs lies in the development of FDTs with controlled release properties. If one FDT can deliver drugs with short half-lives for 12–24 hours, it would be a quantum improvement in the FDT technology.
REFERENCE
1 Cheng R., Guo X., Burusid B., Couch R.A review of fast dissolving tablets. Pharm Tech, (North America). June, 2000; 52-58. 2 Deshmukh VN., Mouth Dissolving Drug Delivery System: A Review, International Journal of PharmTech Research.2012;4(1):412-421. 3 Abdelbary G., Eouani C., Prinderre P., Joachim J., Reynier J., Piccerelle P., Determination of the in vitro disintegration profile of rapidly disintegrating tablets and correlation with oral disintegration. Int.J. Pharm. 2005, 292: 1-2; 29-41. 4 Allen LV., Wang B., Particulate Support Matrix for making a rapidly dissolving tablet. 1997, US patent 5595761. 5 Amin, A.F., Shah, T.J., Bhadani, M.N., Patel, M.M., Emerging trends in orally disintegrating tablets, www.pharminfo.net, 2005. 6 Fu Y, Yang S, Jeong SH, Kimura S, Park K. Orally fast disintegrating tablets: Developments, technologies, taste-masking and clinical studies. The Drug Carrier Sys 2004; 21:433-76. 7 Van Scoik KG. Solid Pharmaceutical dosage in tablet triturates form and method of producing the same. US Patent 5,082, 667. 8 Bodmeier R, 1999. Darreichungsform zur Applikation in Korperoffnungen. German Patent Application DE 19922537. Bradoo R., Fast dissolving drug delivery systems. JAMA India. 2001;4(10): 27-31. 9 Dobetti L. Fast-melting tablets: Developments and technologies. Pharm Technol N Am 2001; Suppl.:44 50. 10 Meyers GL, Battist GE, Fuisz RC. Process and apparatus for making rapidly dissolving dosage units and product there form. PCT Patent WC 95/34293-A1; 1995. 11 Renon, J.P., Corveleyn, S., Freeze-dried rapidly disintegrating tablets, US Patent No. 6,010,719, 2000. 12 Lailla, J.K., Sharma, A.H., Freeze-drying and its applications, Indian Drugs, 1993; 31:503-513. 13 Reddy LH., Fast dissolving drug delivery system: A review of the literature Indian J.Pharm Sci. 2002, 64: 331-336. 14 Debjit B., Chiranjib B., Krishnakanth R., Pankaj, Margret C.,Fast Dissolving Tablet: An Overview Journal of Chemical and Pharmaceutical Research, 2009, 1(1): 163-177. 15 Kuchekar B., Atul S., Badhan C., Mahajan, HS., Mouth Dissolving Tablets: A novel drug Delivery system, Int. J App. Bio. Pharma Tech, 2003; 35: 7-9. 16 Mishra DN., Bimodal M., Singh SK., Vijaya K., and Spray dried excipient base: a novel technique for the formulation of orally disintegrating tablets. Chem Pharm Bull 2006, 54(1): 99-102. 17 Yoshio K., Masazumi K., Shuichi A., and Hiroaki N.,Evaluation of rapidly disintegrating tablets manufactured by phase transition of sugar alcohols. J. Control. Release, , 2005, 105(1-2); 16-22. 18 Vollmer, Paolo Galfetti. Rapid Film: Oral Thin Films (OTF) as an innovative Drug Delivery System and Dosage Form, technology overviews http://www.labtec-pharma.com;Acessed on sept 2007. 19 Seager, H., Drug delivery products and zydis fast dissolving dosage form, J. Pharm. Phamacol., 1998, 50(1-2), 375-382. 20 Fix JA., Advances in quick-dissolving tablets technology employing Wowtab. In IIR Conference on Drug Delivery Systems,October, 1998. 21 Wilson CG, Washington N, Peach J, Murray GR, Kennerley J. Th e behaviour of a fast-dissolving dosage form (Expidet) followed by gamma-scintigraphy. Int J Pharm 1987; 40(1–2):119–123. 22 Sunada H, Bi Y. Preparation, evaluation and optimization of rapidly disintegrating tablets. Powder Technol 2002; 122(2–3):188–198. 23 Sugimoto M, Matsubara K, Koida Y, Kobayashi M. The preparation of rapidly disintegrating tablets in the mouth. Pharm Dev Technol 2001; 6(4):487–493. 24 Dor PJM, Fıx JA. In vitro determination of disintegration time of quick-dissolve tablets using a new method. Pharm Dev Technol 2000; 5(4):575–577. 25 Morita Y, Tsushima Y, Yasui M, Termoz R, Ajioka J, Takayama K. Evaluation of the disintegration time of rapidly disintegrating tablets via a novel method utilizing a CCD camera. Chem Pharm Bull 2002; 50(9):1181–1186. 26 Bi Y, Sunada H, Yonezawa Y, Danjo K, Otsuka A, Iida K. Preparation and evaluation of a compressed tablet rapidly disintegrating in the oral cavity. Chem Pharm Bull 1996; 44(11):2121–2127. 27 Bogner RH, Wilkosz MF. Fast-dissolving tablets: New dosage convenience for patients. US Pharmacist 2002; 27(1):34–43. 28 Susijit S., Mishra B., Biswal PK., Omprakash P., Kumar SM,Kumar GJ., Fast dissolving tablet: As A potential Drug Delivery System.2010;(2): 130-133.









