{ DOWNLOAD AS PDF }
 ABOUT AUHTOR
ABOUT AUHTOR
*R SHARMA, S SARMA
Assam Down Town University, Guwahati, Assam, India
*Rupsmita2010@gmail.com
ABSTRACT
Objectives: The aim of the present study was to estimate flavonoid and phenolic content, and to evaluate invitro antioxidant activity of an aqueous extract of Alpinia nigra and Allium tuberosum.
Methods: The air dried stem of A. nigra and leaves of A. tuberosum was ground to powder and extracted with water and 95% of ethanol. The extract was screened for phytochemicals, total phenolic content (TPC) and total flavonoid content (TFC) with its potential antioxidant activities using hydrogen peroxide-scavenging assay.
Results: Phytochemical test shows that extract contains variety of phytochemicals among which there is a high level of total phenol and flavonoids. The total phenolic content (TPC) of A. nigra and A. tuberosum was 0.450±0.0740 and 1.663±0.296; respectively. The total flavonoid content (TFC) of A. nigra and A. tuberosum was 0.322±0.077 and 0.978±0.119, respectively. The plants possessed potent antioxidant activity when compared with the reference compound ascorbic acid (vitamin C).
Conclusions: A. nigra and A. tuberosum may be useful for the preparation of neutraceuticals as potent antioxidant to treat various human diseases and their complications.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2478
|
PharmaTutor (ISSN: 2347 - 7881) Volume 5, Issue 3 Received On: 04/11/2016; Accepted On: 06/12/2016; Published On: 01/03/2017 How to cite this article: Sharma R, Sarma S;Evaluation of phytochemicals in some indigenous aromatic medicinal plants of North-East India; PharmaTutor; 2017; 5(3); 42-47 |
INTRODUCTION
North east India comprises seven states commonly known as the “Seven Sisters”. These include Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, and Tripura. It is well known for its biodiversity which comprises of various types of flora and fauna.[1] There are over 166 separate tribes. Over generations, these tribes have been using various medicinal plants found in the hill states as home remedies for several types of diseases. Plants containing phytochemicals such as flavonoids and tannins are believed to possess anti-oxidant and anti-inflammatory activities.[2]
Alpinia nigra (Burtt), belonging to the family Zingiberaceae, is widely grown in India, Bangladesh, China and Srilanka. It is also referred to as Jongly Ada, Tara, Galangal, False galangal, Greater galangal, Black-Fruited, or Kala. It is a perennial, rhizomatous aromatic medicinal plant. A. nigra has two flavone glycosides, astragalin and kaempferol-3-O-glucuronide. These two glycosidesare believed to have antibacterial, antioxidant, antiprotozoal, hepatoprotective, and glycation inhibitory effects.[3]
Allium tuberosum belonging to the family Amaryllidaceae is a perennial herb related to onion and mostly grown in China and Thailand for its culinary uses. It is commonly known as garlic chieves, Chinese chieves, or oriental chieves. Essential oil obtained from A. tuberosum has larvicidal activity against larvae of Aedes mosquitoes.[4]
The most important feature of A. tuberosum is considered to be the inhibition of reactive oxygen species (ROS). It is a potential source of several antioxidants and helps control the degenerative or pathological processes involved in aging, cancer, Alzheimer’s disease, and heart diseases.[5]
Plant derived antioxidant compounds act by preventing the generation of free radicals, and thereby alleviate the diseases caused by oxidative stress. However, various synthetic antioxidant agents have been developed to remediate oxidative stress, but factors such as, high cost, lack of availability and side effects remained as major setbacks in combating oxidative stress. Consequently, natural antioxidants received a prominence because they are less expensive, often free from side effects, and abundant in many plant sources.[6]
Therefore, in view of the medical significance, the present study was carried out to screen the phytochemical constituents, to estimate flavonoid and phenolic content, and to evaluate in-vitro antioxidant activity from aqueous extract of the plants, A. nigra and A. tuberosum.
Materials and Methods
Plant materials
A.nigra: Fresh plants free from diseases were collected during the month of January, 2014 from Nalbari district of Assam.
A. tuberosum: The leaves were collected from the market of Manipuri Basti, Guwahati. Taxonomic identification of the plants were carried out in Assam Agriculture University and compared with the herbarium present in the Department of Botany, Cotton College, Guwahati.
The plant materials were thoroughly washed under running water, cut into pieces; air dried for 7 days and pulverized into fine powder in a grinding machine and the resulting fine powder was kept in small plastic bags with paper labeling.
Preparation of water extract
The water extraction was carried out using classical method. The ground leaves material was weighed using an electronic balance and mixed with 100 mL of sterile water (Table 1). After that, the mixture was boiled at 50-60°C for 30 minutes using water bath and it was filtered through WhatmanGrade No.1 filter paper. Then filtrate was centrifuged at 2500 rpm for 15 minutes and filtrate was stored in sterile bottles at 5°C for further use.
Preparation of ethanol extract
Ground samples were extracted with I00 mL of 95% ethanol on water bath at 70°C for two hours (Table 1). The extracted samples were centrifuged at 2500 rpm and the supernatant was transferred into a 50 mL volumetric flask. The residue was further rinsed two more times, the extracts were pooled and the volume adjusted to 50 mL with 95% ethanol. The samples were stored at −4°C until analysis.
|
Table 1: Concentration of the plant extracts prepared |
||
|
Weight (g) |
Volume of water (mL) for the aqueous extracts |
Strength of 100 mL ethanol used |
|
1 |
100 |
95% |
|
2 |
100 |
95% |
|
3 |
100 |
95% |
Qualitative analysis of phytochemicals
Preliminary screening for phytochemicals in aqueous and ethanolic extracts of A. nigra and A. tuberosum plant were done as per standard biochemical procedures as previously described in Arulpriya et al. [3] The phytochemical analysis was done to determine the presence of saponins, steroids, terpenoids, tannins, glycosides, flavonoids, carbohydrates, amino acids, coumarins, anthocyanin and leucoanthocyanins.
Determination of total phenolic content (TPC)
The amount of total phenols in the extracts was determined with the Folin-Ciocalteu reagent method.[7] Gallic acid was used as a standard and the total phenolics were expressed as mg/g gallic acid equivalents (GAE). 1mL of sample was mixed with 1.0 mL of Folin and Ciocalteu's phenol reagent. After 3 min, 1.0 mL of sodim carbonate (7 %) was added and made up to 10 mL by adding distilled water. The reaction was kept in the dark for 90 min, after which its absorbance was read at 725 nm. A calibration curve was constructed with different concentrations of gallic acid (0.01-0.1 mM) as standard. The samples were analyzed in triplicates.
Determination of total flavonoid content (TFC)
Quercetin was used as standard and flavonoid contents were measured as quercetin equivalent. The total flavonoid content was estimated by the aluminium chloride (AlCl3) method. Approximately, 0.5 mL of each sample and 300 μL of sodium nitrite (NaNO2) at 1:20 weight/volume (w/v) were pipetted into a test tube. The contents were vortexed for 10 seconds and left at room temperature for five minutes. Then 300 μL of AlCl3 (1:10 w/v), 2 mL of 1M sodium hydroxide (NaOH) and 1.9 mL of distilled water were added into the mixture. After vortexing for 10 seconds, the absorbance for each sample was measured at 510 nm using UV-visible spectrophotometer. The samples were analyzed in triplicates.
Hydrogen peroxide scavenging assay The ability of the extracts to scavenge hydrogen peroxide (H2O2) was determined according to the method of Nabavi.[8] A solution of H2O2(40mM) was prepared in phosphate buffer, pH 7.4. The concentration of H2O2 was determined by absorption at 230 nm using a spectrophotometer. 4 mL of plant extracts (0.5-3.0gm/mL) in distilled water were added to a H2O2 solution at 230 nm was determined after ten minutes against a blank solution containing phosphate buffer without H2O2. Ascorbic acid was used as the standard compound.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
The H2O2 free radical scavenging activity was calculated by the following equation:
% inhibition = (Acontrol-Atest)/Acontrol × 100
Where control is the absorbance of H2O2 radical+methanol and test is absorbance of H2O2 radical + standard or plant extract
Statistical analysis
The determinations were conducted in triplicate and results were expressed as mean ± standard error. Statistical analyses were done by one-way ANOVA followed by Dunnet’s test with P < 0.05 as a limit of significance. Analysis of variance (ANOVA) test, a statistical analysis was performed which express TPC and TFC as mean ± standard error with P<0.001 as a limit of significance.
Results
The present study was undertaken to screen phytochemicals in aqueous and ethanolic extract of A. nigra and A. tuberosum plants from north eastern part of India. It has been found that saponins and flavonoids were abundant in both plants but steroids were found in trace amount for A. tuberosum while terpenoids and alkaloids were in trace amount in A. nigra. Anthocyanins, leucoanthocyanins, carbohydrates and amino acids were found to be absent in both plants (Table 2). Also the amount of bioactive compouns like phenols and flavonoid in both plants was determined using spectrophotometric analysis.
|
Table 2: Preliminary phytochemical analysis for A. nigra and A. tuberosum |
|||
|---|---|---|---|
|
Phytochemicals screening tests |
Compound |
A. nigra |
A. tuberosum |
|
Froathing test |
Saponins |
++ |
++ |
|
Salkowski test |
Steroids |
++ |
+ |
|
LibermannBurchard’s test |
Terpenoids |
+ |
- |
|
Wagner’s test |
Alkaloids |
+ |
- |
|
Killer-Killiani test |
Glycosides |
++ |
+ |
|
Lead acetate test |
Tannins |
- |
- |
|
Lead acetate test |
Flavonoids |
++ |
+ |
|
NaOH test |
Coumarins |
+ |
- |
|
NaOH test |
Anthocyanins |
- |
- |
|
Isoamyl alcohol test |
Leucoanthocyanins |
- |
- |
|
Ninhydrin test |
Amino acids |
- |
- |
|
Benedict’s test |
Carbohydrates |
- |
- |
The amount of total phenol was determined with the Folin-Ciocalteu reagent method. Gallic acid was used as a standard compound and the total phenols were expressed as mg/g gallic acid equivalent using the standard curve equation: y = 0.006x + 0.038, R2= 0.999, where y is absorbance at 760 nm and x is total phenolic content in the extracts of A.nigra and A.tuberosum expressed in mg/g.
TPC for A. nigra and A. tuberosum was 0.450±0.0740 and 1.663 ±0.296 respectively, when compared with standard reagent gallic acid having 0.243±0.0462 as mean±SE with P < 0.001 as a limit of significance. We found these means were not significantly different (Figure 1).
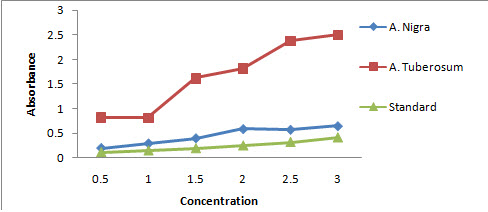
Figure 1: Graph showing absorbance versus concentration for determining total phenolic content
The amount of total flavanoid was determined with the quercetin reagent as a standard compound and the total flavonoid were expressed as mg/g quercetin equivalent using the standard curve equation: y = 0.110x, R2= 0.998, where y is absorbance at 510 nm and x is total flavonoid content in the extracts of A. nigra and A. tuberosum in mg/g. TFC for A. nigra and A. tuberosum was found to be 0.322±0.077 and 0.978±0.119 and compared to standard reagent quercetin whose mean±SE was 15.268±3.33 with P < 0.001 as a limit of significance (Figure 2).
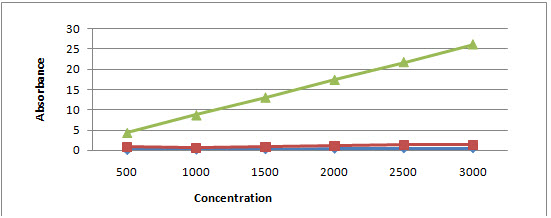
Figure 2: Graph showing absorbance versus concentration for determining total flavonoid content
The extract was screened for its potential antioxidant activities using hydrogen peroxide-scavenging assay.
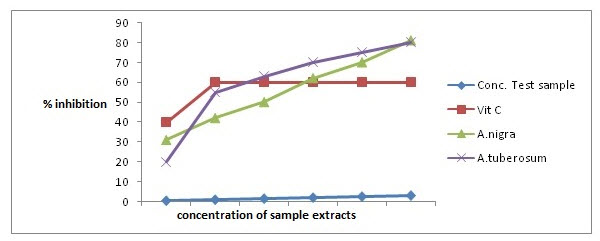
Figure 3: Graph showing percentage inhibition vs conc. of plant extracts to determine antioxidant activity of plants.
Table 3 describes inhibition concentration at 50% of sample concentration. So by plotting the graph of concentration of A. nigra and A. tuberosum vs. percentage inhibition (Figure 3), it has been observed that the aqueous extract of both A. nigra and A. tuberosum scavenges the superoxide radicals upto 80% at 3 g/mL concentration, whereas ascorbic acid (standard) at same concentration scavenged 61%. From the graphical presentation, inhibition concentration (IC50) for A. nigra, A. tuberosum and ascorbic acid was found to be 1.77, 0.84 and 0.66, respectively. This implies that the concentration of A. nigra (1.77) and A. tuberosum (0.84) is needed to inhibit or scavenge the free radicals of H2O2 by half.
|
Table 3: Inhibition cocentration at 50% of sample concentration |
|||
|---|---|---|---|
|
Parameters |
Ascorbic acid (Vit. C) |
A. nigra |
A. tuberosum |
|
IC50 |
0.66 |
1.77 |
0.84 |
Discussion
Phytochemicals are compounds found in plants. Many of these show curative activity against several human ailments and therefore explain the use of traditional medicinal plant for the treatment of some illnesses.
Zingiberaceae family constitutes a vital group of rhizomatous medicinal and aromatic plants characterised by the presence of volatile oils and oleoresins of export value with considerable potential as antimicrobial agents.[9]. Previous phytochemical investigations revealed the presence of alkaloids, flavonoids, tannins, saponins, carbohydrates, quinone, terpenoids, steroids, anthraquinone, phenols, betacyanin, glycosides and proteins in the plant extracts of Samanea saman.[3] The crude form of A. nigra has reportedly been used to cure intestinal helminth infection. The alcoholic extract of A. nigra causes destruction and degeneration of surface archi-tecture of tegument, inhibits energy metabolism related enzymes and also enzyme responsible for neuromuscular co-ordination. [10]
The aqueous extract of A. nigra scavenges the superoxide radicals up to 80% at 3 g/ml concentration, whereas standard ascorbic acid at the same concentration scavenged 61%. Likewise, the aqueous extract of A. tuberosum scavenges the superoxide radicals up to 80% at 3 g/ml concentration, whereas standard ascorbic acid at the same concentration scavenged 61%. The abilities of the plant extract and ascorbic acid to quench superoxide radicals from reaction mixture is reflected in the decrease of the absorbance (Figure 3). At last IC50 values of free radical scavenging activities were determined and shown in Table 3.
A study on Chinese medicinal plants revealed significant levels of phenolics, flavonoids and trace metal contents were found in Ligustrum lucidum, Paeonia suffuticosa, Salvia miltiorrhiza, Sanguisorba officinalis, Spatholobus suberectus, Tussilago farfara and Uncaria rhyncophylla, which correlated well with their antioxidant and anti-inflammatory activities. The antioxidant and anti-inflammatory activities of water extracts of selected Chinese medicinal plants shows 186.9 ± 0.71, 183.4 ±1.41, 195.4 ±1.41, 99.93 ± 4.04 , 7.09 ± 4.64, 8.22 ± 0.44 , 16.66 ± 1.96, 41.4 ± 5.87.[6]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
One study also reports that the ethanolic extract of A. tuberosum contain higher amount of polyphenolic contents in comparison to water extract. During the evaluation of antioxidant activity, maximum scavenging was observed in case of ethanolic extracts of A. tuberosum with minimum IC50 values 0.736 mg/ml and 0.651 mg/ml against 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and 2,2'-azinobis-(3- ethylbenzothiazoline-6-sulfonic acid) (ABTS)radicals.[5]
In a study by Gopalakrishnan V.K. et al., it was reported that the ethanolic extract of the plant Mollugo nudicaulis showed higher antioxidant activity due to the presence of phenols, flavonoids, tannins, carotenoid and lycopene.[11]
The extract of A. nigra and its bioactive compound astragalin showed dose dependent anthelmintic effect against Fasciolopsis buski. Anthelmintic efficacy of plant bioactive compound is mediated through changes in the vital tegumental enzymes, like acid-and alkaline phosphatase, and changes in the surface ultrastructure of the parasite.[12]
Since flavonoids and phenols seems to be most promising polyphenolic compounds for protection of living systems, it can be concluded that both A. nigra and A. tuberosum having phytochemicals as flavonoid and phenols could be useful for the preparation of neutraceuticals as potent antioxidant to treat various human diseases and its complications.
Conclusion
Phytochemical analysis of medicinal plants have been found to possess many bioactive compounds, hence proved that plants, traditionally used for medicinal purpose have effective chemical properties for treating illness. Analysing phytochemicals for antioxidant activity provides clear evidence that how medicinal plants effectively lead to the development of new medicines with lesser side effects.
Thus, the present study shows that the selected indigenous plants containing flavonoids and phenols have strong anti-oxidant activity. So, it can be concluded that both plants A. nigra and A. tuberosum could be used for treating various human diseases.
Acknowledgement
The authors would like to thank the team at PFC Pharma Focus India Pvt Ltd for its support in reviewing and editing the manuscript.
References
1. Seven Sisters State of India. Available from: http://www.indiatravelguru.in/2015/10/seven-sisters-of-india.html. Accessed on: July 11, 2016.
2. Zhang Yu-Jie., Gan Ren-You., et al; Antioxidant Phytochemicals for the Prevention and
Treatment of Chronic Diseases; Molecules; 2015; 20; 21138–21156.
3. Arulpriya, P. Lalitha, S. Hemalatha; Competence of different solvent extraction methods in the isolation of bioactive principles from Samanea saman (Jacq.) Merr; International Journal of Pharmaceutical Sciences; 2012; 2(2); 184-196.
4. Saxena M., Saxena J., Nema R., Singh D., and Gupta A; Phytochemistry of Medicinal Plants; Journal of Pharmacognosy and Phytochemistry; 2013; 1(6); 168-182.
5. Sultana F., Mohsin M., Sah AN; In-vitro Antioxidant and Antimicrobial Activity of Allium tuberosum Rottler. ex Spreng; Int. J. Adv. Res. Biol. Sci.; 2015; 2(12); 178–187.
6. Ravipati SA., Zhang L., Koyyalamudi RS., Jeong CS., et al; Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content; BMC Complementary and Alternative Medicine; 2012; 12;173.
7. Stanly C., Bhatt A., Ali HMD., Keng CL., Lim BP; Evaluation of free radical scavenging activity and total phenolic content in the petiole-derived callus cultures of Zingiber zerumbet Smith; J Med Plant Res; 2011;5(11);2210–2217.
8. Nabavi SM., Ebrahimzadeh MA., Nabavi SF., Hamidinia A., Bekhradnia AR; Determination of antioxidant activity, phenol and flavonoids content of Parrotia persica mey; Pharmacology online; 2008a; 2:560-67.
9. Nahak G., Sahu RK.; Evaluation of antioxidant activity in ethanolic extracts of five curcuma species; International Research Journal of Pharmacy; 2011; 2(12); 243-248.
10. Roy B., Swargiary A., Giri BR.; Alpinia Nigra (Family Zingiberaceae): An Anthelmintic
Medicinal Plant of North-East India; Advances in Life Sciences; 2012, 2(3); 39-51.
11. Gopalakrishnan V.K. et al.; Radical Scavenging and Antioxidant Activity of Ethanolic Extract of Mollugo nudicaulis by Invitro Assays; Ind J Pharm Edu Res; 2011; 45; 4.
12. Swargiary A., Roy B.; In Vitro Anthelmintic Efficacy of Alpinia nigra and its bioactive compound, astragalin against Fasciolopsis buski; Int J Pharm Pharm Sci; 2015; 7(10); 30-35.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









