{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
SHIVANAND *, SAYANTAN MUKHOPADHAYAY
Division of Pharmaceutical Sciences S.G.R.R.I.T.S
Dehradun, Uttarakhand 248001, India.
*shiva3671@gmail.com
ABSTRACT:
The active pharmaceutical ingredient that is thermolabile and moisture sensitive in nature generally degraded in atmospheric condition and thus have reduced stability and self-life. Lyophilization is one of those techniques which is utilized effectively to improve such critical condition. It is the one of the emerging technology in themodern era, which is effectively involved in the preparation of several antibiotics (e.g., chloramphenicol, doxycycline) and anti-cancer drugs (e.g., doxorubicin, epirubicin). This technique effectively utilized the phenomenon of sublimation to obtained primary dried product followed by removal of excess amount of moisture by modulation of heat. This technique not only improved self-life of thedrug but also provides fast reconstitution and reduced the cost of storage and shipping. Inthis review article principle behind lyophilization, steps involved, formulation aspects, theimportance of lyophilization and detection of the end point in lyophilization along with recent advancementwas explained.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2535
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 11 Received On: 10/07/2017; Accepted On: 14/07/2017; Published On: 01/11/2017 How to cite this article: Shivanand A, Mukhopadhayaya S; A Review on Lyophilization: A Technique to Improve Stability of Hygroscopic, Thermolabile Substances; PharmaTutor; 2017; 5(11); 28-39 |
INTRODUCTION
Lyophilization or Freeze-Drying Technique
Lyophilization is the most common technique for the manufacturing of parenteral pharmaceutical product when the product is unstable in aqueous solution. It is helpful in long-term storage and makes the product stable, for ensure the stability of sterile product require low moisture content about less than 1% for the preservation of the pharmaceutical product. Lyophilization or freeze drying (FD) mainly involved in the removal of solvent (water) from the pharmaceutical product. In this process, thefirst Solvent is in a frozen state (solid) and then water is removed by sublimation process in a vacuum chamber. The Lyophilization technique mainly consists of three unique, separate, & interdependent processes; freezing, primary drying (sublimation), and secondary drying (desorption).[1, 2, 3]
The use of physical phenomenon for sublimation, which mainly involves the direct conversion solid state (ice) to the gaseous state. Freeze-drying is an effective technique for drying the pharmaceutical product without changing the product properties. In the lyophilized parenteral formulation, water is used as a main component. Hydrolysis is the most common instability mechanism of parenteral. In as much as, lyophilization technique is widely used for preservation of sensitive molecules from degradation (example- water sensitive &thermolabile) and improves long term storage. The final product makes easy reconstitution and restoration to solution form.Lyophilizationis a drying process used in the manufacture of pharmaceuticals and biologicals product those are thermolabile or unstable in aqueous solutions and make the product storage for a prolonged time. [4, 5]
Characteristics, advantages and disadvantages of Lyophilized Product[6, 7, 8]
Characteristics
• Long stability of the product.
• Minimal reconstitution time.
• Elegant cake appearance.
• After reconstitution maintains original dosage form characteristics, including solution properties; structure and conformation of a pharmaceutical product, proteins, and particle-size dispersion in suspensions.
• Isotonicity is maintained after reconstitution of the product.
Advantages
• Chemical decomposition is minimized.
• Without excessive heating water is removed from the product.
• In a dry condition enhances product stability.
• Dissolution is fast for reconstituted products.
• From thermosensitivematerial, water can remove without changing the molecule's properties.
• A lyophilized product having the high specific surface area, which helps rapid and complete rehydration of the solid.
• In vials, freeze-dried dosage forms filled as a solution, which makes more easy and precise filling than powder in to vials.
Disadvantages
• The requirement of sterile diluents for reconstitutionof the product.
• Many pharmaceutical drug and biological products such as vaccines, liposomes, and protein are damaged by freeze-drying during process time.
• Stability of a drug in the solid state mainly depends on its physical state of molecules.
• Time increases for processing and Handling.
• By using vacuum method volatile removed from the product.
• Processing time, Cost and complexity of equipment increased
Applications of lyophilization
There are several applications of lyophilization or freeze-drying method used in the manufacturing of pharmaceutical and a biological product. Lyophilization is used in the pharmaceutical industry, for the production of
• Injectable drug product.
• Solid oral dosage forms (Example- Ondansetron-rapid orally dissolving tablets).
Methods of Lyophilization
Depending on the type of product requirement different drying technique may be used. Several different lyophilization methods are respectively
• Manifold drying
• Batch drying
• Bulk drying
Manifold drying
In this lyophilization methods, froze solid (ice) vials or flasks individually attached to the drying chamber ports. This method is generally used for products having a high collapse or eutectic temperatures with small volumes. The advantage of this method is that since each vial or flasks direct connected with the collector. Hence, the drying process is relatively faster as compared to the other drying method and enhance the drying efficiency. Since each vial or flask is attached individually to the drying chamber, it can be removed from drying chamber without interrupting the other flasks or vials.[ 9]
Batch drying
In this lyophilization methods, used for the drying of a large number of vials having similar size and contain the same drug product. It having the potential to the control of temperature and pressure. Since all the vials were kept at the similar drying chamber (controlled temperature and pressure), the variability of the product is minimized. This batch drying method is widely used in the pharmaceutical industry.[10]
Bulk drying
In this lyophilization methods, the product is directly poured in a single unit in a tray and placed the tray in a drying chamber, this method is known as bulk drying. The product doesn’t uniform dried. Hence, the product doesn’t lend sealed in the aseptic area. Usually after removal of the product from equipment packed in air tight containers. This drying process is generally for those products that are not sensitive to moisture or oxygen.[11]
Principle behind freeze drying
The main principle behind freeze drying process is known as sublimation, in the sublimation process water is directly converted from solid state (ice) to the vapor phase without goes into a liquid state. At below triple pointsublimation of water can take place at particular temperature and pressures. The liquid to be first frozen solid (ice) and frozen solid to be placed in a high vacuum chamber to heat (by using radiation or conduction or by both). Hence frozen liquid sublimes leaving behind the only solid product or a dried product of the original liquid. The driving force for removal of water during lyophilization is the concentration gradient of water vapor between the drying front and condenser.
To remove the water from formulation, the lyophilization or freeze-drying process consists of;
• Froze the formulation into solid (ice).
• By sublimation process, the solid (ice) directly converted into vapor by using a vacuum chamber.
• Remove the water vapor by using a condenser. After removal of water from the formulation, the freeze-dried product can be removed from the machine.
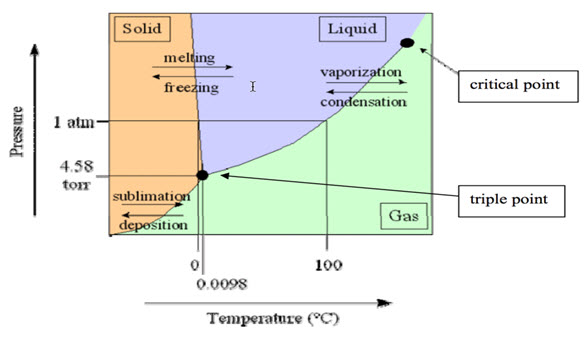
Fig .1:Phase diagram of water triple point.[39]
The triple point of any substance is the temperature and pressure at which the three phases (solid, liquid, and gas) of that substance coexist. The phase diagram of water shown in Fig. 1. Water have three physical states at a particular temperature and atmospheric pressure (example-pressure 0.611657 kPa or mbar 0.00603659 atm & temperature 273.16 K or 0.01 °C).[1, 7, 12] The principle behind freeze or lyophilization is sublimation is based on its physical phenomena. The solid (ice) in the product is directly transformed into water vapor phase without going into a liquid state. If the partial water vapor pressure is lower than the partial pressure of the solid (ice) then the solid become avapor at this relevant temperature.[13]The vapor pressure over ice chart data shown in Table. 1.
|
Vapor pressure over ice chart |
||||||
|
Temperature |
Vapor Pressure |
Temperature |
Vapor Pressure |
|||
|
Deg C |
mTorr |
mBar |
Deg C |
mTorr |
mBar |
|
|
0 |
4,584.00 |
6.111480 |
-50 |
29.500 |
0.039330 |
|
|
-2 |
3,883.00 |
5.176893 |
-52 |
23.000 |
0.030664 |
|
|
-4 |
3,281.00 |
4.374295 |
-54 |
17.900 |
0.023865 |
|
|
-6 |
2,765.00 |
3.686353 |
-56 |
13.800 |
0.018398 |
|
|
-8 |
2,325.00 |
3.099737 |
-58 |
10.600 |
0.014132 |
|
|
-10 |
1,949.00 |
2.598446 |
-60 |
8.100 |
0.010799 |
|
|
-12 |
1,630.00 |
2.173149 |
-62 |
6.160 |
0.008213 |
|
|
-14 |
1,359.00 |
1.811846 |
-64 |
4.660 |
0.006213 |
|
|
-16 |
1,130.00 |
1.506539 |
-66 |
3.510 |
0.004680 |
|
|
-18 |
936.80 |
1.248960 |
-68 |
2.630 |
0.003506 |
|
|
-20 |
774.40 |
1.032446 |
-70 |
1.960 |
0.002613 |
|
|
-22 |
638.20 |
0.850861 |
-72 |
1.450 |
0.001933 |
|
|
-24 |
524.30 |
0.699007 |
-74 |
1.060 |
0.001413 |
|
|
-26 |
429.40 |
0.572485 |
-76 |
0.780 |
0.001040 |
|
|
-28 |
350.50 |
0.467294 |
-78 |
0.570 |
0.000760 |
|
|
-30 |
285.10 |
0.380101 |
-80 |
0.410 |
0.000547 |
|
|
-32 |
231.20 |
0.308240 |
-82 |
0.290 |
0.000387 |
|
|
-34 |
186.80 |
0.249045 |
-84 |
0.210 |
0.000280 |
|
|
-36 |
150.30 |
0.200383 |
-86 |
0.150 |
0.000200 |
|
|
-38 |
120.60 |
0.160786 |
-88 |
0.100 |
0.000133 |
|
|
-40 |
96.30 |
0.128389 |
-90 |
0.072 |
0.000096 |
|
|
-42 |
76.70 |
0.102258 |
-92 |
0.049 |
0.000065 |
|
|
-44 |
60.80 |
0.081060 |
-94 |
0.034 |
0.000045 |
|
|
-46 |
48.00 |
0.063995 |
-96 |
0.023 |
0.000031 |
|
|
-48 |
37.70 |
0.050262 |
-98 |
0.015 |
0.000020 |
|
Steps involved in freeze drying process
The freeze-drying process mainly consists of three stages.Fig. 2 demonstrate that the steps involved in freeze drying process.
• Freezing
• Primary drying
• Secondary drying
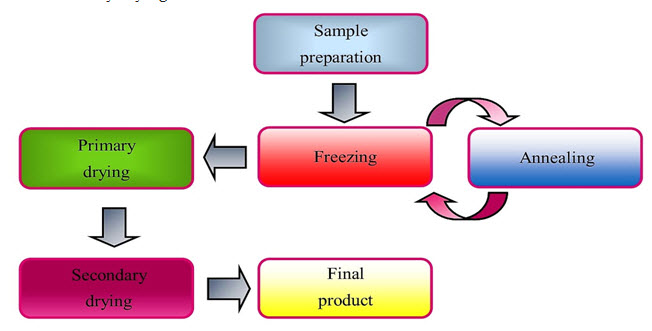
Fig. 2: Steps involved in the process of freeze drying.[1]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Freezing
The freezing is the first stage in lyophilization it's very simple to the concept but is the least understood & most complex step. The optimization & careful control of the freezing process determines the proficiency with which drying process occurrence and the type of final product formed. In freezing process, the solvent, generally water is frozen to form ice. The solute which presents in the solid (ice) stays in the interstitial space between the ice crystals. The solid (ice) is removed during the primary drying steps.[14]The freezing process is a critical step because the microstructure of the ice crystals formed in freezing will subvene the final product of quality and influence the primary and secondary drying process.[15]
There are three physical phenomena occur during freezing.
Super cooling
The main process of freezing is to assure that all the vial content is completely converted into a constant solid form (ice). Freezing of solution failure depends on the Freezing point depression & lack of nucleation centers.The existence of concentrated pockets of the solution will lead to a tumble of freezing point. These may stay in liquid state. There is no formation of crystallization can take place (example-no nucleation formation), the cooling process is not sufficient for the freezing of solution. For the freezing of product required super cooling i.e., the temperature should be must lower than the ice formation temperature for the complete freezing process to occur.
Primary crystallization
The Primary crystallization is the formation of crystals & nucleation of the solvent is known as primary or ice crystallization.
Secondary crystallization
The concentration of the solutes during the formation of crystallization & ice crystal growth is known as secondary crystallization. After the formation of crystal growth and crystallization, the vial content becomes completely solid. The complete freezing depends on the characteristics of solvent, solute, and temperature. The two factors that are important during freezing such as temperature & time of freezing and cooling rate.[16]
Temperature & Time of Freezing
Temperature & Time of Freezing is one of the important factors in the development of lyophilization product, eutectic temperature (Teu) for crystalline substances & glass transition temperature (Tg) for amorphous substances. During development of the lyophilizedproduct, freezing temperature should be below the eutectic temperature (Teu) or glass transition temperature (Tg) for assuring that complete freezing occurs.
For freezing requires significant time because thermal conductivity is limited between the shelf and the glass vial. By using trial and error method optimum time for freezing is determined. The depth filling in vials also plays an important role in freezing. As the filling of the productis increased, the time required for freezing also increased.[17]
Cooling Rate
Theformation of smaller & large ice crystals depends on its cooling rate. By rapid cooling rateresult in the formation of small ice crystals.But theformation of asmall ice crystal is difficult to dry as compared to large ice crystals. In pharmaceutical industries for the formulation of lyophilized injections, rapid drying is important. If thecooling rate is slowerthat’s leads to the formation of larger ice crystals which are easy to dry.
Annealing
The process involved in annealing is to control thecooling& heating rate of the product and to stimulate crystallization. Annealing is step involved within the freezing process. Usually, annealing is used to crystallize the bulking agent and helpful in the formation of large ice crystals during the freezing process. The annealing process is helpful in an alienate variation of initial ice crystal size distributions induced by a variable in nucleation (crystallization) temperatures and the resulting in heterogeneity drying rates. Lyophilization or freeze drying is very important for the development of high-value pharmaceuticals product. Thus, consistency of the product is essential. Variation and position of vials kept in the lyophilizer chamber will result in differences in the formation ice nucleation leads to product quality and drying rate heterogeneities.[17]
Some amorphous products (such as glycine or mannitol) which show incomplete crystallization when it first froze in a metastable glass. These products can obtain complete crystallization by using a thermal treatment process, which is also known as annealing. At the time of annealing process, the product temperature is cycled from -40℃ to -20℃ within few hours and then it back to -40℃ to obtain a complete crystallization. Annealing has the added the various advantage in the lyophilization technique of larger crystal growth and shorter drying times.Hence reduces the primary drying time and improves the dissolution rate of the lyophilized product & make the good cake appearance.[18, 19]
Primary Drying
After freezing next step in the lyophilization is the primary drying. In this primary drying sublimation of ice (solid), formed during the freezing (example- by the process of sublimation the ice transform into vapor phase without pass through the liquid phase. The vapor formed during sublimation is removed by condenser plates.
The three basic necessity of primary drying that is involved;
• Chamber pressure,
• Target product temperature,
• Shelf temperature,
The primary drying is done in a such a way in which sublimation process occurs from the frozen product, resulting in a dry and structurally intact lyophilized product formed. Sublimation of ice starts from the surface of the frozen material and continues to the bottom of the vial during the primary drying process.[17, 20]
Chamber pressure
Molecules always migrate from the region of high pressure to low pressure. The chamber pressure is less than that of the ice vapor pressure at given temperature of the product. The variable of the chamber pressure, which alter the process of heat and mass transfer. This influence the sublimation rate. The ideal chamber pressure range is 50-200 mTorr for the process.
Target product temperature
For obtained desirable product & structurally elegant product, the content of vial should not have collapsed during the process of primary drying. For intercept collapse, it is very necessary to set the target product temperature. The target product temperature must be set below several degrees than collapse temperature (Tc) of the product. The temperature difference amongst the target product temperature & the collapse temperature is knowing the temperature safety margin. The temperature of the product high to get a faster drying rate. Hence, an optimized cycle used for a target product temperature which is high as possible, but the temperature of the product must below the collapse temperature. During the primary drying process safety margin temperature, must be set to get a protection from the collapse of the product. The temperature safety margin, 2 ̊ C as a small safety margin is recommended cycle time (less than 2 days), intermediate safety margin at 3 ̊ C cycle between (10 hours to 2 days), high safety margin 5 ̊ C for a short cycle (less than10 hours).
Shelf temperature
The energy required for the process of sublimation is got from the heat provided by the shelf of the drying chamber, shelf temperature is an important parameter for the drying process and it determines the product temperature. Determination of an optimal shelf temperature, time profile is one of the essential steps in freeze drying process it is helpful to get the desired target product temperature. The shelf temperature is always higher than the vial temperature, during the primary drying process. The range for temperature difference is (5 to 40 ̊ C).
Determination of the End Point of Primary Drying
Freeze drying is a time and energy consuming process, it takes longer time for sublimation of ice. For reduce the time of secondary drying by increasing the shelf temperature of primary drying. For the determination of the end point of primary drying helpful in shortening the overall cycle time and it also reduces the cost and process time. The temperature probe or thermocouple used to determine the end point of primary drying outer vials. The temperature of the vial is same as that of the shelf, hence no heat is required for the process of sublimation. But this is not denotative of all the vials temperature. Therefore, the probe is placed in the outer vial for check weather product is dried or not. This is difficult for those vials which are placed on the interior side of the chamber. When it confirms the end point of primary drying, then it safe to add an additional soak time (at least 10-20%) of the primary drying time to ensure that sublimation of ice has been completed.[21]
Techniques used for determination of gas composition in the product chamber;
• Pirani gauge
• Measuring water concentration using tunable diode laser absorption spectroscopy(TDLAS)
• Lyotrack
Others include:
• Product thermocouple response
• Condenser pressure
• Manometric temperature measurement
Pirani Gauge
Pirani gauge is an instrument used for the measurement of thermal conductivity of the gas in the drying chamber. In process of primary drying involves conversion ice into (sublimation) vapor. Therefore, during primary drying chamber contains mostly water vapor. This vapor condenses by using a condenser. When the process of sublimation is completed, in the chamber presence of nitrogen starts to increase vapor falls. Then end point is determined by the difference in the thermal conductivity.[21]
TDLAS
Tunable diode laser absorption spectroscopy (TDLAS) is helpful in the determination of sublimation rate as well as water vapor concentration. It is mainly consisting of two laser beams, one of them is directed towards the vapor flow &another one against the vapor flow in the duct connected with chamber and condenser. The laser beam is adjusted in to the absorption lines of the gas in the duct, (example water vapor). This determines the concentration of vapor.[21, 22]
Lyotrack
Lyotrack is also called as the gas plasma spectroscopy. Lyotrack is mainly working on the principle of optical emission spectroscopy. It is helpful for the determination of water vapor concentration.Lyotrackmainly consists of a plasma generator. It generates radio frequency & energy is transmitted into the plasma tube filled with gas. Absorbed energy leads to converted the gas into plasma.[23]
Problems raises with Lyophilization
In the formation of cake during lyophilization process, two common problems occur;
• Product collapse
• Melt back
Product collapse
Product Collapse during freeze drying product temperature exceeds the collapse temperature and the material “collapse” as ice is sublimed.After ice sublimed a dried residue of solute is produced.
Melt back
Melt back can generally occurfrom collapse which changed in state from solid to liquid.[24, 25]Fig. 3 shown the schematic diagram of product collapse.
Fig .3:Examples of schematic diagram product collapse during primary drying.[40]
Secondary Drying
Secondary drying, we also knew as the process of desorption, removal of water from the product. This process of desorption is helpful in remove the bound water or water adsorbed on the internal surface of the product. The water content of the product is low enough that there are no chemical reactions or biological growth occurs. Therefore, secondary drying is done for reducing the water level and removed the residual water and ensure the stability of the final product. This total process depends upon the materials adsorption isotherm. Fig. 4 shows that the bound water present in primary drying get removed in the secondary drying. Secondary drying (desorption) is a slower process than primary drying albeit the quantity of water removed is very less in secondary drying. As compared to primary drying, secondary drying takes longer time for desorption. In the product, low moisture content level is acceptable and the slow desorption process, result required a longer time for drying. Research demonstrated that in secondary drying water level is very low on the surface of the cake and the walls of the vial compared than rest of the cake. This happens due to the shrinking of the cake from the wall of the vial. This helpful to provides a path for the escape of water vapor. In spite of, storage of vials under normal conditions found to be a uniform distribution of moisture everywhere among the cake. If shrinkage of the cake does not happen, then the lowest moisture content occurs at the top of the cake.[17, 20]

Fig .4: Examples of bound water present in primary drying get removed in the secondary drying.[40]
Parameters that occurs during Secondary Drying process
The parameters that occur in secondary drying are similar to primary drying;
• Target product temperature
• Shelf temperature
• Chamber pressure
Target Product Temperature
The final temperature of the product after secondary drying is the target product temperature or (Tp). Determination of target product temperature is depending upon two factors, secondary drying time and the stability of the drug at the final temperature. The target product temperature must be set in such a way that the drug does not degrade when exposed at temperature for a prolonged period of time and the temperature should be enough that the desired level of moisture content should be attained. Hence, reducing the drying time, the desired water content is not attained and thus it affected the stability of the product. The product that is crystallinity in nature, it is very important for the determination of the secondary drying time. For the product that is amorphous in nature, having high water content and that require a longer time for secondary drying time than the crystalline products.[26]
Shelf Temperature
The shelf temperature is the source of energy, it is used for removal of water in secondary drying. Secondary drying (desorption) requires lesser energy than compared to primary drying, during the process of desorption shelf temperature should not increase too much. But, the temperature should be enough that to ensure removal of the unbound water from the product in a short period of time without degradation of the product.
Chamber Pressure
The pressure of drying chamber should be maintained constant for ensuring that the moisture content present in the vial should be removed from the vial.
Heat Transfer Rate
Heat transfer rate is useful in the determination of the time for the drug or product exposed at a particular temperature. The rate of heat transfer is depending upon the amount of moisture content present in the cake after primary drying. If the water (moisture) content at the end of primary drying is high, a low rate of transfer of moisture content (Rs) therefore exposure of the drug product to heat for a long period of time for removing the moisture content resulting in degradation of the drug. In that cases, the rate of heat transfer should be increased for ensuring thermal stability of the product.
In the secondary drying of amorphous products, heat transfer rate plays a major role in removing of moisture content. The moisture content present in the amorphous products is high and have a low transition temperature, therefore required a high rate of heat transfer might result in the collapse of the cake. Hence, heat transfer rate must be kept as low as possible for amorphous drugs products. With crystalline drugs product, this problem has not been seen and rates of heat transfer high are allowed. [27, 28]
The fig.5 schematic diagram shows that the heat and mass transfer that occurs in the drying chamber. The diagram demonstrated that, due to the direction of heat and mass transfer rate the vial of top product to dry first then drying proceeded downward to the bottom of the vial. Hence, the drying process exists in a three-component or layer system in each vial.
• The upper dry product
• The middle sublimation fronts
• The lower frozen liquid product

Fig .5: The schematic diagram shows that the heat and mass transfer that occurs in the drying chamber.[1]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Evaluation parameter for Lyophilized Products[29,30]
Appearance
The cake should not be collapsed on removal from the lyophilizer chamber. It must occupy the same volume at the of the liquid filled before the lyophilization or freeze-drying process.
Reconstitution Time and Clarity of Reconstituted Solution
In the lyophilization process when the surface area of the cake increases, resultant solubility also increases. Reconstitution of the product should take a very least time as possible and after reconstitution completion, the solution should be clear, without any visible particles in solution.
Water Content
The water content present in the productis determined by Karl Fischer titration method. The water content present in lyophilization product is very less. Water determination test is mainly used to perform the water content present in the substances. In this process,there is aquantitative reaction between water, iodine and sulfur dioxide in the presence of a methanol (alcohol) and pyridine (organic base).
Assay
The assayis based on either a chromatography purity assay or a microbial bioassay. In which substance of unknown purity or activity is compared with a reference standard with precisely determined purity or bioactivity. The assay is either reported as “Assay (on the dried basis)” or “Assay (as is).”
Bio-assay is mainly used to testing a substance of unknown potency against certain microorganisms. How the substance was at inhibiting microbial growth and compare the potency with areference standard.
Purity assay ismainly determined by comparing HPLC peak responses of the substance and compared with the appropriate reference standards. Assayon “on dried basis” or “anhydrous” are calculated without water content. Assayon “as is” are calculated with water content.
Stability
The stability test is the main concern for lyophilization, water content present in product affected the stability of the product. The long-term stability studies and at accelerated stability should be done as per ICH guidelines, placing the vials instability chamber.
Sterility
The lyophilized product is reconstituted for parenteral use, Water for Injection (WFI) or any other sterile solvent can be used for reconstitution of the product. To ensure that the product should be sterilized by performing sterility testing.
Excipients selection for lyophilized formulation
The excipients selection for the lyophilized formulation depends on the active pharmaceutical ingredient (API) and route of administration. The lyophilized formulation may contain one or more excipients that perform a specific function. [31, 32, 33]
Excipients may be used as a;
• Buffers
• Bulking agents
• Stabilizers
• Tonicity modifiers
Buffers
Buffers used in pharmaceutical formulations for stabilizing the pH. The choice of the buffer is critical in the development of lyophilized formulation, sodium phosphate, and Phosphate, buffers, undergo drastic pH changes during the freezing process. The low concentrations use of citrate and histidine buffers that undergo minimal pH change during the freezing process.[34]
Bulking agents
The bulking agent used in the lyophilized product to provide bulk to the formulation. when very low concentrations of the active ingredient (API) are used. The bulking agents those are crystalline in nature provide good mechanical properties with elegant cake structure. [35]
Stabilizers
Stabilizers in lyophilization formulation, used as disaccharides form an amorphous sugar to be used for stabilizing products such as proteins and liposomes during the process of lyophilization. Trehalose and Sucrose are inert and have been used in stabilizing protein, liposomes, and various formulations. Glucose, maltose, and lactose are reducing sugars and used in prevent of proteins from the mallard reaction. [36, 37]
Tonicity adjustors
An isotonic formulation required is required several cases. The tonicity adjuster required in a formulation for the stability of the bulk solution or in the case of the route of administration. Excipients such as glycerol, sodium chloride, mannitol, glycine, and sucrose are good tonicity adjusters. Glycine is used as lower the glass transition temperature in the amorphous phase. [38]
CONCLUSION:
The lyophilized technique is used in the development of stable injectable dosage form for a drug those having poor self-life and stability problem degraded in the presence of moisture content present in the formulation. By using freeze drying technique the stability and self-life of the product are enhanced. The drypharmaceutical product is mainly prepared by lyophilization. There are following step involved in the lyophilization: freezing, primary drying,and secondary drying.In freezing process, the solvent, generally water is frozen to form ice. The solute present in the interstitial space between the ice crystals. Primary drying mainly involved sublimation of ice that transforms into vapor phase without going through the liquid phase. The vapor formed during sublimation process removed by condenser plates in lyophilization chamber. In the secondary drying water content of the product is low enough that there are no chemical reactions or biological growth occurs. Therefore, secondary drying is done for reducing the water level and removed the residual water and ensure the stability of the final product. Approximately 50% of biopharmaceuticals product are lyophilized, it representing the most common formulation technique. In lyophilization freezing, thestep will help to improve more accomplished lyophilization cycles and biopharmaceutical products with an improved stability.
REFERENCES:
1. Sandip K., Rajesh K.and Mallinath H; A review on freeze drying process of pharmaceuticals; Int J Res Pharm Sci;2013; 4(1); 76-94
2. Suruchi S.V; A review on rational design to optimize stable lyophilized parenteral products; Int J Pharm Sci Rev Res;2014; 29(2);169-178
3. Girish PK., Vibha V and Sreenivasa M; Lyophilization: An emerging trend in formulation ofparenterals; IJPRS; 2014; 3(3);393-402
4. Nail SL; Fundamentals of freeze-drying Development and manufacture of protein pharmaceuticals;Marcel Dekker; 2002; 281–360.
5. Nireesha GR., Divya L and Sowmya C; Lyophilization freeze drying: A review;IJNTPS; 2013; 3(4);87-98
6. Tushar RJ and Moon RS; Review on lyophilization technique; WJPPS;2015; 4(5);1906-1928
7. Sunil R., Ashutosh B and Ritesh V; A review: The lyophilization process for the development of unstable drug;EJBPS; 2015; 2(4); 1137-1151
8. Pradnya M.K., Bhushan B and Saudagar R.B; Lyophilization Technique: A Review; Asian J. Res. Pharm. Sci;2016;6(4); 269-276
9. Chang B.S and Patro S.Y; Freeze-drying process development for protein pharmaceuticals Lyophilization of Biopharmaceuticals;AAPS; 2004; 113-138
10. Xiaolin T and Michael J.P; Design of freeze-drying processes for pharmaceuticals practicaladvice;Pharm Res;2004; 21(2); 191-200
11. Frank Kofi BA; Understanding lyophilization formulation development; Pharm. Tec; 2004; 20; 10-18
12. Bisht D and Zeenat I; Lyophilization Process and optimization for pharmaceuticals;IJDRA;2015; 3(1);30-40
13. Lippincolt and Williams K; Remington thescience & practice of pharmacy Parenteral preparation; ISE publication; 2000; 1; 804-819
14. Kasper JC and Friess W; The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals; Eur. J. Pharm. Biopharm;2011; 78(2);248-263
15. Avis KE., Lieberman HA and Lachman L; Pharmaceutical dosage forms Parenteral medications; Marcel Dekker; 1993.
16. Searles JA., Carpenter JF and Randolph TW; The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature controlled shelf;J. Pharm. Sci;2001; 90(7);860-71
17. Searles JA., Carpenter JF and Randolph TW; Annealing to optimize the primary drying rate, reduce freezing-induced drying rate heterogeneity, and determine Tg′ in pharmaceutical lyophilization; J. Pharm. Sci; 2001; 90(7); 872-887
18. Hawe A and Friess W; Physicochemical characterization of the freezing behavior of mannitol-human serum albumin formulations;AAPS; 2006; 7(4); 85-94
19. Lian Y., Nathaniel M and Edward G.G; Existence of a mannitol hydrate during freeze-drying and practical implications;J. Pharm. Sci;1999;88(2);196-198
20. SadikogluH., Liapis A. I and CrosserO. K; Optimal control of the primary and secondary drying stages of bulk solution freeze drying in trays, drying technology; An International Journal; 1998; 16(5); 399-431
21. Sajal M.P., Takayuki D and Michael J.P; Determination of end point of primary drying in freeze-drying process control; AAPS; 2010; 11(1); 73-84
22. William J.K., Michael F and Steven J.D; Evaluation of tunable diode laser absorption spectroscopy for in‐process water vapor mass flux measurements during freeze drying;J. Pharm. Sci;2007; 96(7); 1776-1793
23. Y. M and R.V; Freeze-drying process monitoring using a cold plasma ionization device;PDA J Pharm Sci Technol; 2007;61; 160-174
24. Koustuv C., Evgenyi Y.S and Raj S; Partially crystalline systems in lyophilization II withstanding collapse at high primary drying temperatures and impact on protein activity recovery;J. Pharm. Sci; 2005; 94; 809-820
25. Wallen AJ., Van Ocker SH and Sinacola JR; The effect of loading process on product collapse during large-scale lyophilization;J. Pharm. Sci;2009; 98(3); 997-1004
26. Chang BS., Beauvais RM and Carpenter JF; Physical factors affecting the storage stability of freeze-dried interleukin-1 receptor antagonist: glass transition and protein conformation; Arch. Biochem. Biophys; 1996; 331(2); 249-58
27. Bardat A; Moisture measurement a new method for monitoring freeze-drying cycles; J Parenter Sci Techno; 1993; 47(6); 293-299
28. Wei W; Lyophilization and development of solid protein pharmaceuticals; Int. J. Pharm; 2000; 203; 1-60
29. Sunil R and Ashutosh B; The lyophilization process for the development of unstable doxorubicin injectable formulation;IAJPS;2015;2(9); 1339-1347
30. Mikkilineni R., Srinivasa P.B and Mandava V.B.R; Formulation and evaluation of stable lyophilized bendamustine hydrochloride injection; Int J Pharm Sci Rev Res; 2013;23(2); 89-93
31. Neema S., Washkuhn RJ and Brendel RJ; Excipients and their use in Injectable products; PDA J Pharm Sci Technol; 1997; 51; 166-171
32. Shah N.V., Solanki H and Prajapati V; Impact of formulation ingredients on qualityof the parenteral products; WJPPS; 2015; 4(3); 468-482
33. Shah R and Mehta P; Freeze dried injectable drug product development selection of non-functional additives; Int J Pharm Pharm Sci;2014; 6(9): 3-7
34. Shireesh P.A and Sydney O.U; A review and classification of emerging excipients in parenteral medications;Pharm Tech; 2003; 46-60
35. Nema S and Brendel RJ; Excipients and their role in approved injectable products current usage and future directions; PDA J Pharm Sci Technol; 2011; 65(3); 287-332
36. Sougata P., Deepak S and Vikas C; Excipient selection in parenteral formulation development; Pharma Times;2013; 45(3);65-77
37. Yasir M and Umer F; Excipients use in parenteral and lyophilized formulation development; Open science journal of pharmacy and pharmacology; 2015; 3(3); 19-27
38. Ankit B., Lokesh K and Arvind K.B; Excipients used in lyophilization of small molecules; J. Excipients and Food Chem; 2010; 1(1); 41-54
39. Freeze Drying / Lyophilization Information: Basic Principles [Internet]. Spscientific.com. 2017 [cited 10 June 2017]. Available from: http://www.spscientific.com/freeze-drying-lyophilization-basics
40. Pharmaceutical Freeze Drying: The Lyophilization Process [Internet]. 2010 [cited 10 June 2017]. Available from: https://www.ispe.org/great-lakes/milton-lyophilizatio-2010-presentation.pdf
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









