{ DOWNLOAD AS PDF }
ABOUT AUTHOR
Manimala M*, Vijay Kumar D
Santhi ram College of Pharmacy
Kurnool, Andhra Pradesh, India
*manimala.mosey@gmail.com
ABSTRACT:
The word “forensic” means “pertaining to the law”; forensic science resolves legal issues by applying scientific principles to them. Forensic Analysis is the application of Analysis to law enforcement or the failure of products or processes. It is unique among chemical sciences in that its research, practice, and presentation must meet the needs of both the scientific and the legal communities.Forensic Analysis applied the basic theory and mythology of advanced analytical chemistry to rationalize various materials of crimes and provide evidence to detection.Forensic Analysis is carried out by the two types of techniques namely Organic Analytical Technique &In- Organic Analytical Technique. And this review article covers regarding how Analytical techniques are useful in forensic analysis to resolve major problems of common man.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2545
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 12 Received On: 12/08/2017; Accepted On: 16/08/2017; Published On: 01/12/2017 How to cite this article: Manimala M, Vijay KD;A review on Forensic Analytical Chemistry; PharmaTutor; 2017; 5(12); 12-17 |
INTRODUCTION:
Forensic Analysis is the application of Analysis to law enforcement or the failure of products or processes As such, forensic Analysis research is applied and derivative by nature and design, and it emphasizes of metrology and validation Department forensic analytical chemistry includes forensic science and technology and forensic chemistry. Forensic Science can be viewed as a tripartite structure consisting of a Collection, which pertains to the science investigation, Examination, which pertains to the medical investigation and Presentation, which pertains to the courts.
Forensic science is applied science, often called “criminalistics” Forensic science applies biology, physics, geology, chemistry to civil and criminal laws and places physical evidences into a professional discipline. Forensic science applies the knowledge of technology of science for the definition and enforcement of law.
Forensic science is the use of advanced scientific techniques during criminal and civil investigations. Forensic science can answer important questions about a any crime and be applied to legal cases that are civil in nature. Forensic science plays an integral role in the criminal justice system.
Forensic Analysis is carried out by the two types of techniques.
- Organic Analytical Technique
- In Organic Analytical Technique
Organic Analytical Techniques:
The organic analytical techniques are carried out for the analysis of organic
compounds such as Alcohols, Aldehydes , Alkenes, Amines, Carboxylic acids, Esters, Ketones, Phenols etc. in the unknown substances. The analysis provides the identity (qualitative result) and the amount (quantitative result). Various analytical techniques used for analysis of organic compounds are
- Chromatography
- UV- visible Spectrophotometery
- Infrared Spectrophotometery
- Mass Spectrometry.
- Gas Chromatography-Mass Spectroscopy
- Liquid Chromatography-Mass Spectroscopy
Inorganic Analytical Techniques:
These inorganic analytical techniques are carried out for the analysis of inorganic
elements such as C, B, NA, MG, AL, SI, P,K, CA, TI, CR, MN, FE, NI, CU, ZN, SR, S, AG, SN,Ba, Pb,
V,MO etc. in the unknown substances. The analysis provides the identity (qualitative result) and the amount (quantitative result).
Various analytical techniques used for analysis of organic compounds are
Atomic Absorption Spectrophotometry
Neutron Activation Analysis
X-Ray Diffraction Analysis
Flourimetry
NephloTurbidimetry
Atomic Emission Spectroscopy
The flow of forensic analysis [4] :
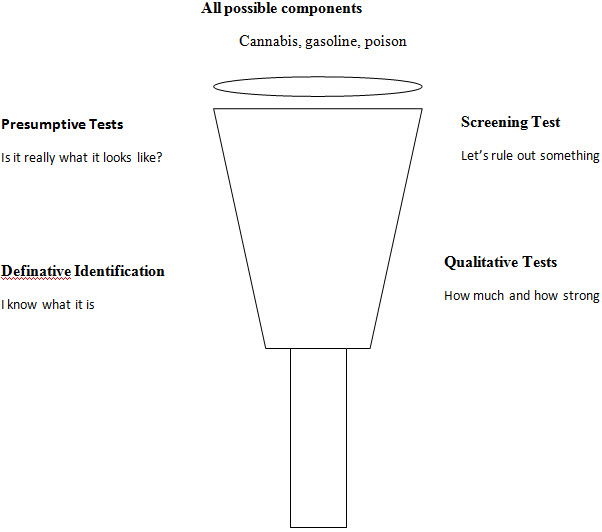
Evidences used in forensic [5,6,7,8,9,10] :
Hair:
Hair analysis to ascertain the presence of illegal drugs and heavy metals in the body, and DNA testing, among others . . Toxic substances may be 200-300 times more highly concentrated in hair than in blood or urine. Because of its tough outer coating, hair does not easily decompose. Therefore, hair is the tissue of choice for detection of toxic substances.
Finger nails:
Finger nails have individualizing striations on them as the result these are used in a forensic analysis
Body fluids:
Body fluids are divided into two type’s excreted fluids and secreted fluids. Excreted fluids: The excreted fluids that are found at crime scene include feces, vomit, bile and sebum
Secreted fluids:
The fluids are divided into two type’s excreted fluids and secreted fluids. Excreted fluids: The excreted fluids that are found at crime scene include feces, vomit, bile and sebum
Urine :
Urine analysis is carried out for Alcohol and Drugs testing since Drugs and drug metabolites found in urine are usually stable. Drugs and their metabolites are often present in higher concentrations in urine than in other biological materials. Drug and their metabolities detectable in urine for relatively long period of time Common Drugs Analyzed in Hair, Bodyfluids, finger nails&urine are :Nicotine Cotinine Amphetamine Alcohol Barbiturates Heroin Tobacco LSD. CommonTrace Elements Analyzed in Hair are: Iron Copper Mercury Calcium Bromine Cobalt Potassium Zinc Selenium
Drugs and Trace elements are analyzed by: HPLC, Mass Spectroscopy, Laser technique Uv-Visible Spectroscopy,I.R Spectroscopy.
Fiber:
Fiber is the most common and integral piece of evidence located at a scene of crime.Fiber Analysis is usually magnified in cases of homicide, assault, or sexual offences.
Paint :
Forensic Paint Analysis deals with analysis of all forms of paint layers to investigate fraudulent claims on vehicle crashes. Paint is made up of three main components, the carrier, the binder and vehicle. Trace elements analyzed in paint: Lead, Cadmium, Chromium, Manganese, Nickel, Aluminum, Copper Mercury, Cobalt ,Zinc,Selenium
Glass:
Glass can be used as evidence in crimes ranging from burglaries, RTA accidents, murder, assault, ‘ram-raids,' criminal damage and thefts of motor vehicles Trace elements analyzed in glass are:Sio 2 ,B 2 o 3 ,Fe 2 o 3 ,Zro 2 ,Al 2 o 3 ,Na 2 o,K 2 o,Na 2 co 3
Finger prints:
Finger prints can be left on almost any physical objects or surfaces and when left in the right conditions they can remain there for several years. Each fingerprint is unique to an individual and no two finger prints have ever been found to be the same.
Footwear and Footprint:
The print left behind at crime scene can give vital evidence in forensic analysis. This are analyzed by:Raman Spectroscopy,Light Microscopy,I.R Spectroscopy,Mass Spectroscopy,Uv-Visible Spectroscopy,FTIR Spectroscopy,Gas Chromatography,GC- MS,Stereo Microscopy Chemical Reagents like ninhydrin reagent, diazafluorenone
Types of Samples [11,12] :
Samples used for analysis are of two types:
Control samples:
The known samples used in analysis are called as control samples
Questioned samples:
The evidence samples used in analysis are called questioned samples
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Presumptive tests:
These tests are used for preliminary identification of sample
Confirmatory tests:
These tests are used to make a more specific identification of sample
Both tests carried out by:
- Gas Chromatography (GC)
- Mass Spectrometry (MS)
- High Performance Liquid Chromatography (HPLC)
- Thin Layer Chromatography (TLC)
- Immunoassays
- Atomic Absorption Spectroscopy
- Atomic Emission Spectroscopy
- Inductively coupled plasma emission (ICP/AES)
- Mass Spectrometry
- Scanning Electron Microscopy (SEM)
- Fourier Transform Infrared Spectrometry (FTIR)
- Ultraviolet Spectrometry (UV/Vis)
- Electrophoresis
APPLICATION OF VARIOUS ANALYTICAL TECHNIQUES USED IN
FORENSIC ANALYTICAL CHEMISTRY [17,18] :
They are two types of analytical methods Destructive methods: Destructive methods involves the destruction of sample and looses the integrity of sample Ex: spectroscopic & chromatographic techniques
Non-Destructive methods:
This techniques conserves the integrity of the sample since they do not involve in destruction of sample Ex: optical microscopy and micro spectroscopy
Compound light microscope:
The compound light microscope is probably the most widely used microscope today. This microscope has a light source and multiple lenses to obtain high magnification.The compound microscope usually has a magnification between 40× (40 times) and 1,000× (1,000 times). Compound microscopes are powerful enough to view hair, fibers, and cells
Stereomicroscope:
The light of a stereomicroscope, or dissecting microscope, is reflected from the surface of the specimen. Because the light is reflected, the stereomicroscope produces a three-dimensional image useful for dissecting. Surface details are also more visible with the stereomicroscope. Stereomicroscope is used to examine insect larvae, paint chips, and other small items of evidence.
Comparison microscope:
The comparison microscope is actually two microscopes connected to one eyepiece. The image on the right is of the specimen under the microscope on the right and can be compared side-by- side to the image on the left.The comparison microscope is particularly useful when comparing bullet striations, fibers, and hair samples.
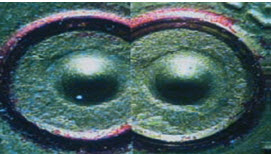
Images of two bullets casings as seen through a comparison microscope

Comparison microscope
Electron microscopy:
Electron microscopes use beams of electrons to form images. These microscopes can magnify materials up to 500 with good resolution, but the image is in black and white. The transmission electron microscope (TEM) passes a beam of electrons through a thin slice of a specimen. This produces images of internal structures. The scanning electron microscope (SEM) passes a beam of electrons over the surface of a sample to produce a three-dimensional image. This image provides details about the surface of the sample. Forensic investigators use electron microscopes to analyze small specimens and to view tiny surface or internal details. SEM is also an important step in the determination of the identity of trace materials, such as gunshot residues
Chromatography:
Most analytical techniques can be classified as either quantitative or qualitative. A quantitative analysis will result in a measurable amount a quantity. Qualitative analysis, on the other hand, will result in a description or identification of the components of a mixture. Qualitative tests are based on the physical and chemical properties of the sample. Chromatography separates substances within a mixture based on their physical properties Different substances will adhere, or stick, to solid surfaces or dissolve in a solvent differently. In paper chromatography, a small amount of a liquid mixture is placed near the bottom of a piece of paper. The RF value is a qualitative comparison between the length of time the substance is in the mobile phase and in the stationary phase. In paper chromatography, the RF value is the ratio of the distance the substance traveled to the distance the solvent traveled.
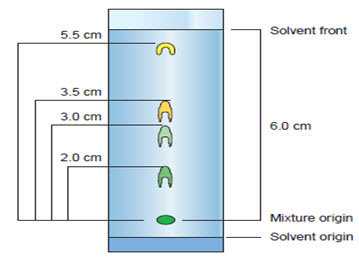
Chromatogram used for calculations RF value
RF = Distance travelled by solut
Distance travelled by solvent
= 3cm\6cm =0.5
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Chromatography is used in forensic science to analyze dyes in fibers, test for explosives or accelerants, and to check body fluids for the presence of drugs.More sophisticated forms of chromatography have replaced paper chromatography.
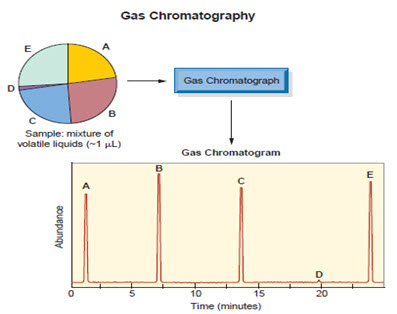
Gas chromatography helps to determine the components of a mixture each spike represents different component of mixture .The relative heights tell relative abundance of each component. Thin-layer chromatography (TLC), gas chromatography (GC), and high- performance liquid chromatography (HPLC) are commonly used in forensic laboratories. Unlike GC, HPLC can take place at room temperature.Therefore, HPLC can be used to test for the presence of flammable materials, such as explosives or accelerants.
Spectroscopy: When a substance is heated, it emits light at a specific wavelength. Electromagnetic spectroscopy uses this chemical property to determine what elements are present in a sample. Spectroscopy can be used to identify fibers and other trace evidence. It can also be used to detect contaminants in various materials. Spectroscopy can detect accelerant and explosive residue. An electromagnetic spectrograph measures the wavelengths of light emitted and captures a spectral image on photographic film. The spectral image is a series of lines.Each element produces a unique line in the spectral image. So, the pattern of lines tells the scientists which elements are found within the sample. Spectroscopy also measures the amount of light absorbed, which can be used to determine the concentrations. There are several forms of spectroscopy. Mass spectroscopy is often combined with gas chromatography to identify atoms and molecules by their masses.A sample is loaded into the mass spectrometer and vaporized and ionized, forming charged particles called ions. The ions are then sent through a magnetic or electric field. The path of the ion depends on the ratio of its mass to its charge.The results are recorded on a photographic plate. Every chemical has a unique mass spectrum, making mass spectroscopy useful as a confirmatory test. Atomic absorption spectroscopy (AAS) measures the amount of light of a specific wavelength absorbed by atoms of a particular substance.This technique is especially useful in determining heavy-metal contaminants in air, water, and soil samples. It is also useful when analyzing paint chips. This technique can help forensic scientists determine whether soil or paint at the crime scene can be linked to another location.Ultraviolet (UV) spectroscopy measures wavelengths of light and can be used to determine the concentration of different elements in a solution. The graph produced by UV spectroscopy is compared to that of known substances as part of a quantitative analysis of the data. UV spectroscopy can be used to detect drugs in blood or urine, analyze components of dyes and food additives, and monitor air and water quality.
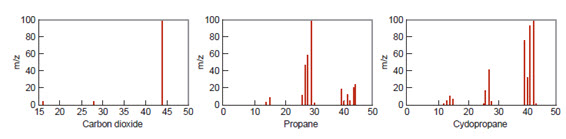
Spectrographs of carbon dioxide, propane, and cyclo propane.
CONCLUSION:
In simple terms forensic Analysis can be put across as a study and application of Analytical principles to matters of law. The hair analysis is carried out by HPLC and presence of drugs like nicotine and cocaine ,body fluid analysis is carried out by the G.C and AAS and presence of alcohol and various trace elements are determined. Forensic analysis evidence is invaluable to the criminal justice system. Forensic Analysis has undergone dramatic progress in recent years, including in the area of DNA collection and analysis and the reconstruction of crime scenes. Finally the scope of forensic analysis increases broadly in dealing with many criminal cases and I wish my review forensic article may helpful to deal such cases..
REFERENCES:
[1]Teaching Forensic analytical chemistry by William E.Brewer(1), Stephen J.
Lambert(2),Stephen L.Morgan(3),and Scott R.Goode(3)
[2]Introduction to Forensic science by Cavanaugh.
[3] H. J. M. Bowen, Trace Elements in Biochemistry.
[4] www.biologymad.com/cells/microscopy.htm servation and forwarding of biological samples for toxicological analysis in Medico legal autopsy cases: A review Tabin Millo, A.k jaiswar and C.Behera.
[5] "Drugs of Abuse Reference Guide," labcrop Inc Retrieved online April 11, 2007.
[6 ] Trace Evidence Analysis,by Houck, Max M (Ed),Academic Press (2001).
[7] P. Kintz, I. Kieffer, J. Messer and P. Mangin, Nicotine analysis in neonates’ hair for measuringGestational exposure to tobacco. J. Forensic Sci., 38 (1993) 119-123.
[8] P. Kin@ B. Ludes and P. Mangin, Evaluation of nicotine and cotinine in human hair. J. ForensicSci., 37 (1992) 72-76.
[9] A. Mizuno, T. Uematsu, A. Oshima, M. Nakamura and M. Nakashima, Analysis of the nicotine content of hair and its utilization for assessing individual cigarette-smoking behavior. There. Drug Monit. 15 (1993) 99-104.
[10] T. Uematsu, Therapeutic drug monitoring in hair samples. Clin. Pharmacokinetic. 25
(1993) 83-87.
[11] S. Pichini, R. Pacifici, I. Ahieri, A.R. Passa, M. Rosa and P. Zuccaro, Analysis of nicotine and cotinine in human hair by high-performance liquid chromatography and comparative determination with radioimmunoassay. In NIDA Research Monograph ‘Hair Testing for Drugs of Abuse: International Research on Standards and Technology’. NIH Publication No. 95-3727, 1995, pp. 212-224.
[12] M.J. Welch, L.T. Sniegoski, C. All good and M. Habram, Hair analysis for
drug abuse: evaluation of analytical methods, environmental issues, and development of
reference materials. J. Anal. Toxicol. 17 (1993) 389-398.
[13] R. Pacifici, S. Pichini, I. Ahieri, M. Rosa, A. Bacosi, A. Caronna and P. Zuccaro, Determination of nicotine and two major metabolites in serum by solid-phase extraction and high-performance liquid chromatography and high-performance liquid chromatography-particle beam mass spectrometry. J.Chromatogr. 612 (1993) 209-213.
[14]Robert H. Garrison and Elizabeth Somer, The Nutrition Desk Reference, Keats
Publishing Co New Canaan, CT; 1985.
[15] Jean A. T. Pennington and Helen Nichols Church, Food Values of Portions
Commonly Used, 13th ed., J. B. Lippincott Co.: Philadelphia; 1980.
[16] Bernice K. Watt and Annabel L. Merrill, Handbook of the Nutritional Contents of Foods, Dover Publications, Inc.: New York; 1975.Determination of Calcium by AAS
[17]Lerner, K. Lee, and Brenda Wilmoth Lerner, Eds. (2006) the world of forensic science. New York:
[18]Newton, Michael. (2008) .The encyclopedia of world science. New York: Info Base Publishing, pp. 125–126.Siegel, Jay. (2006)Forensic science The Basics. Boca Raton, FL: CRC Press, pp. 317–318
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









