About Authors:
Sukhbir L.Khokara1*, Naveen2, Jitendra2, Balram2, Dhirender2, Rajender Kumar1
1Department of Pharmacy, Manav Bharti University, Solan (H.P.) 173229
2Institute of pharmaceutical sciences, Kurukshetra University, Kurukshetra, Harayana-136119
rajkaushal13@gmail.com
ABSTRACT
These heterocyclic moiety are prepared by different method and have different biological and physiological properties. The widespread use of 1,3, 4 –oxadiazole as a scaffold in medicinal chemistry establishes this moiety as an important bioactive class of heterocyclic compounds. These compound have biological properties like anticonvulsant, analgesic, antipyretic, antimitotic,antitubercular and antimicrobial etc.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1315
INTRODUCTION:
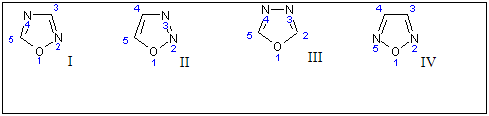
Oxadiazole ring is considered to be derived from furan ring by replacement of two methane (-CH=) group by two pyridine type nitrogen (-N=) [1]. There are four possible isomers of oxadiazole (I, II, III and IV)depending on the position of nitrogen atom in the ring and are numbered as shown in.
Heterocyclic compounds bearing 1, 3, 4-oxadiazole moiety has been used as a pi-conjugation relay to prepare a number of donor-acceptor molecules carrying a pi-electron rich aromatic ring. Therefore the compounds bearing 1 ,3, 4-oxadiazole moiety may be a good candidate for optical material or biologically active chemicals [2].
1, 3, 4-oxadiazoles have attracted an interest in medicinal chemistry as ester and amide bioisosteres for a number of biological targets [3, 4].
As such their peptidomimetic ability has been explored and reported in the development of Phe-Gly mimetics of dermorphin (Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2) and substance P(Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2) [5].
The widespread use of 1, 3, 4-oxadiazoles as a scaffold in medicinal chemistry establishes this moiety as an important bioactive class of heterocycles. These molecules are also used as pharmacophores due to their favourable metabolic profile and ability to engage in hydrogen bonding [6]. 1, 3, 4-oxadiazoles have proved to be useful in material science as probe for their fluorescence and scintillation properties [7].
In addition oxadiazole derivatives have been widely used as electron conducting and hole blocking material in molecules based as well as polymeric light emitting devices [8].
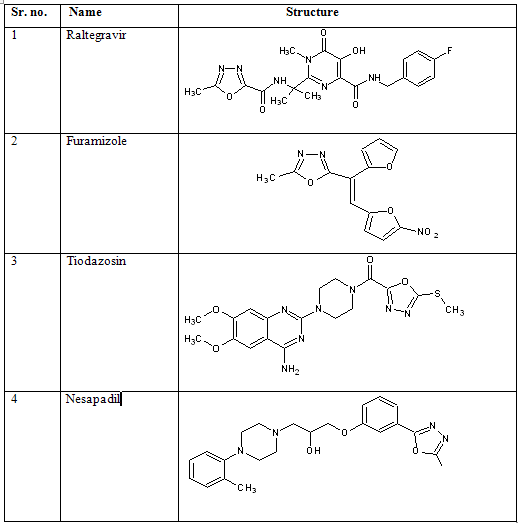
Oxadiazole, a heterocyclic nucleus has attracted a wide attention of the chemist in search for the new therapeutics. Out of its four possible isomers 1, 3, 4-oxadiazole is widely exploited for various applications. A number of therapeutic agents such as HIV-integrase inhibitor raltegravir [9], a nitrofuran antibacterial furamizole [10], antihypertensive agents tiodazosin [11], and nesapidil [12] are based on 1,3, 4-oxadiazole moiety.
Drugs containing 1, 3, 4-oxadiazole moiety
1. Chemistry of oxadiazole:-As 1,3 ,4-oxadiazoles have a relatively low electron density at carbon (position 2 & 5) and a relatively high electron density at nitrogen (position 3 & 4), the major reactions are nucleophilic attack at carbon, generally followed by ring cleavage, and electrophilic attack at nitrogen. This
reactivity towards nucleophiles, also catalysed by acid, causes difficulties when carrying out reactions which involves basic or acidic conditions. The ring is more stable when substituted by one or more aryl groups [13].

Tautomeric oxadiazoles (a&b) react with electrophiles at ring nitrogen, at the exocyclic heteroatom or at both centres. Reactions in the subsstituent group of alkyl or aryl-1, 3,4-oxadiazoles are possible but they are limited by sensitivity of the ring to the reagent used.
1.1. Fully conjugated Rings; Reactivity at Ring Atoms:-
[adsense:468x15:2204050025]
1.1.1.Thermal and photochemical rections, formally involving no other species:-
1,3,4-oxadiazoles are thermally stable compounds and this stability is increased on substitutions, particularly by aryl and perfluoroalkyl groups. Oxadiazolin-5-ones and 5-thiones are somewhat less stable and undergo selective pyrolysis at high temperatures.
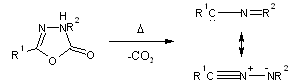
Oxadiazolinones (1) lose carbon dioxide at high temperature to give nitrilimines (2) which react further. Recrystallization in the nitrilimine, formed at 210-230?C from oxadiazolinone (1a), yields a 2-alkoxy-1,3,4-oxadiazole which at the reaction temperature, undergo a further rearrangements to oxadiazolinone (1c). A similar rearrangement occurs on thermolysis of oxadiazolinone (1b). 2,5-diphenyl-1,3,4-oxadiazole is produced on heating oxadiazolinone (1d) at 250?C. Oxadiazolinones (1e) & (1f) are more stable but undergo flash vacuum pyrolysis at 500?C to give indazoles. At higher temperature, loss of nitrogen also occurs, and styrene or fluorine respectively is produced in high yield. A variety of hydrocarbons are formed on flash vacuum pyrolysis of oxadiazolinones (1; R2= alkenyl).
|
a |
R1 |
R2 |
|
b |
Ph |
COOR |
|
c |
Ph |
COSEt |
|
d |
Ph |
R |
|
e |
Ph |
COPh |
|
f |
Me |
Ph |
|
g |
Ph |
Ph |
Carbon dioxide is lost on thermolysis of 4-ethoxycarbonyl-2-phenyl-Δ2-1, 3, 4-oxadiazoline-5-thione. Migration of the ethyl group from oxygen to sulfur leads to the product, 5-ethylthio-2-phenyl-1,3,4-oxadiazole.Photolysis of oxadiazolinone (1f) yields a nitrilimine (2f) which, in the presence of alkenes, undergoes cycloaddition to give pyrazoles. Azobenzene is produced on irradiation of 3,4-diphenyl-1,3,4-oxadiazolidine-2,5-dione.
1.1.2. Electrophilic attack at nitrogen:-
Alkyl and aryl-1,3,4-oxadiazoles are neutral compounds and 2-amino derivatives are weak bases. Protonation is believed to occur at ring nitrogen in position 3 which facilitates ring cleavage in aqueous acid. The pka (water) value of 2-amino-5-methyl-1, 3, 4-oxadiazole is 2.37 and values in the range 2.3-2.7 have been recorded for 2-amino-5-phenyl- and 2-N-methylamino-5-phenyl-1,3,4-oxadiazole in 50% aqueous ethanol. In the same solvent pka values of the more basic imines, 4-methyl-2-phenyl- and 4 N-dimethyl-2-phenyl- Δ2-1,3,4-oxadiazolin-5-imine, are 6.3 and 6.38 respectively.
Alkylation of oxadiazolines (3) and aminooxadiazole (4) generally result in substitution at ring nitrogen in position 4 or 3 respectively, particularly under neutral conditions. In alkaline medium, alkylation at the exocyclic heteroatom may occur

Oxadiazolinones (3a) and thiones (3b) acylate, for example, with acetic anhydride, benzoyl chloride or alkyl chloroformates at ring nitrogen in position 4.
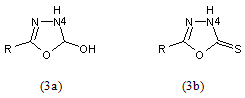
2,5-disubstituted 1,3,4-oxadiazoles form double salts with mercuric chloride or with silver nitrate, which in the latter case, are used to purify or characterize the oxadiazole.
1.1.3. Electrophilic substitution at carbon:-
The relatively low electron density at carbon, coupled with the possibility of Protonation at nitrogen, makes electrophilic substitution at carbon difficult. A further problem is acid catalyzed ring cleavage, particularly with alkyl-oxadiazoles. No example of nitration or sulfonation has been reported.
1.1.4. Reaction of nucleophiles at carbon:-
The attack of a nucleophiles at carbon leads either to nucleophilic displacement (path a) or ring cleavage (path b), the latter being the most common result. Treatment of 2-chloro (4) or 2-Methyl sulfonyl-oxadiazoles with amines, theorem or azide ion to yields the corresponding 2-substituted oxadiazoles (5).
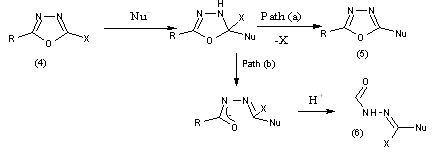
The most frequently encountered result of the reaction of a 1,3,4-oxadiazoles with a nucleophiles is ring opening to a hydrazine derivative (6). This may undergo further reaction such as hydrolysis, or recrystallization to a 1,2,4-triazole (7) where x or nu is an amino group.

Alkyl and aryl-1,3,4-oxadiazoles undergo acid or base catalysed ring opening in water. Susceptibility of hydrolysis increase with solubility. Hence alkyl-oxadiazoles ring opening is more readily than aryl-oxadiazole and 2,4-diaryl-1,3,4-oxadiazoles are fairly stable in dilute acid or alkali at 100?C
Oxadiazolinones undergo ring opening in hot water to form hydrazine carboxylic acids (8) which decarboxylate to acylhydrazines. These acyl hydrazines may subsequently attack the ring of the starting oxadiazole causing cleavage to 1,5-diacylcarbonohydrazides. Oxadiazolinethiones are more resistant to nucleophilic attack and thiones is stable in hot water.
1.1.5. Nucleophilic attack at hydrogen; Acidity:-
Deprotonation at C-2 or C-5 has not been reported. Oxadiazolin-5-ones (9) and -5-thiones (10) are weak acids and formally undergo deprotonation at the NH group. pKa values in the range 6.6-6.7 have been quoted for oxadiazolinones and a slightly higher pKa of 7.93 has been quoted for 2-methyl- Δ2-1,3,4-oxadiazolin-5-one. Oxadiazolinethiones are stronger acids, having pKa values of 3.85, 4.45 and 4.27 respectively.

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2. SYNTHESIS OF 1,3,4-OXADIAZOLE BACKBONE:-
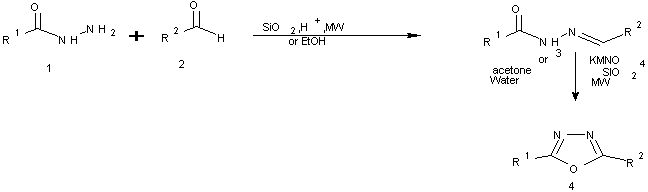
A series of 2,5-disubstituted 1,3,4-oxadiazoles were prepared by oxidation of 1-aroyl-2-arylidine hydrazides with potassium permanganate on the surface of silica gel and also in mixture of acetone and water under microwave irradiation[14].
Scheme 1 synthesis of 2,5-disubstituted 1,3,4-oxadiazoles using microwave
2-phenyl-5-trimethylsilyl-1,3,4-oxadiazole reacted with Cl2 or Br2 in hexane at 00 C for 0.25-1.5 hr affording the corresponding 2-halogen-5-phenyl-1,3,4-oxadiazoles which ppt from the reaction mixture and were isolated in good yield[15,16].
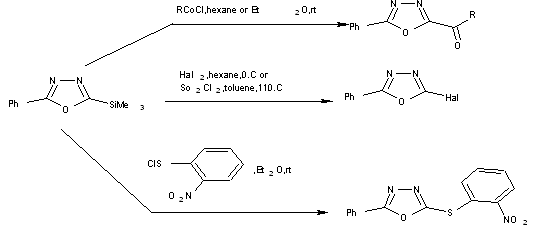
Scheme2 synthesis of 2,5-disubstituted 1,3,4-oxadiazoles using trimethylsilyl.
Acid hydrazides were coupled with acrylic acid derivatives and cyclodehydration gave 1,3,4-oxadiazoles lastly in situ nitrile oxide formation from aryl oximes treated with sodium hypochlorite, and subsequent 1,3-dipolar cycloaddition to the exomethylene moiety delivered 2-(4,5-dihydroisoxazol-5-yl)-1,3,4-oxadiazoles[17,18].
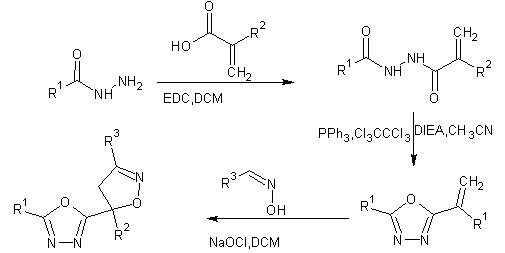
R1= 4-Cl-C6H4, C6H5, Benzyl
R2=CH3, H
R3= 4-Pyridyl, 4-F-C6H4, 3-Pyridyl
Scheme 3 synthesis of 2-(4,5-dihydroisoxazol-5-yl)-1,3,4-oxadiazoles
2,5-disubstitutes 1,3,4-oxadiazoles have been conveniently prepared by oxidative cyclization of N-acyl N`aryliden-hydrazines promoted by excess of Dess Martin reagent(1,1,1-triacetoxy-1,1-dihydro-1,2-benziodoxol-3(1H)-one[19].
Scheme4 2,5-disubstituted 1,3,4-oxadiazole from Dess Martin Reagent
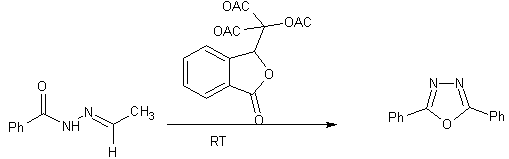
1,3,4-oxadiazoles can be prepared from carboxylic acid and acid hydrazides with PS-PPh3/CCl3CN [20]
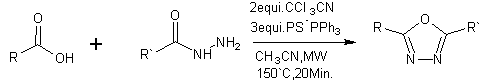
Scheme 5 Formation of 1,3,4-oxadiazoles using PS-PPh3 resin combined with microwave heating.
Reaction of 1-benzoyl-5-hydroxy-pyrazoline with ketene, prepared in situ from the corresponding acid chloride and mixed anhydride result in formation of 1,3,4-oxadiazoles [21].
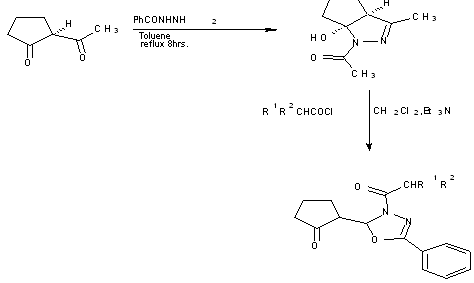
Scheme 6 Formation of 1,3,4-oxadiazoles from reaction of N-aroyl dihydrocyclopenta-pyrazolidinol with ketene.
A series of novel glucose based 3-acetyl-5-alkyl-2, 3-dihydro-1,3,4-oxadiazoles withtheassistance of microwave was developed. The effect of different catalysts on the heterocyclization process was investigated and the reaction conditions were optimized with NaOAc emerging as the catalyst of choice. Under the optimized condition a series of novel3-acetyl-5-alkyl-2,3-dihydro-1,3,4-oxadiazole derivatives were successfully synthesized[22].
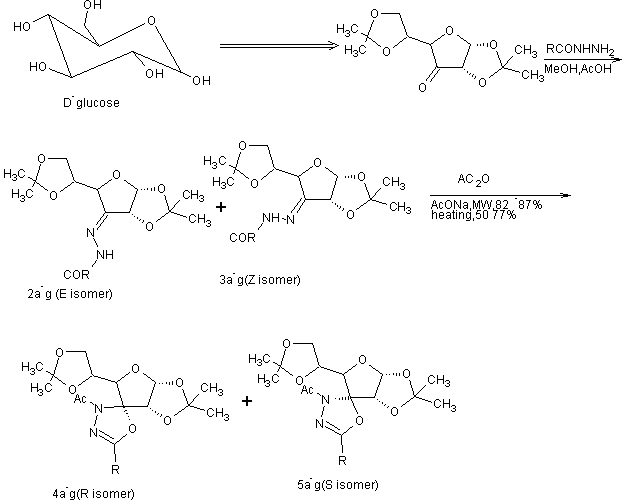
Scheme 7. Microwave assisted synthesis of glucose based 3-acetyl-5-alkyl-2,3-dihydro-1,3,4-oxadiazole derivatives.
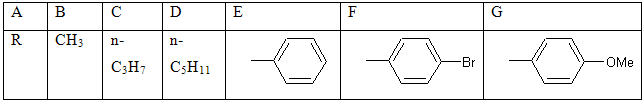
The cycloaddition of substituted ylidene-N-phenylhydrazine-carbothioamides with 2,3-diphenylcyclopropenone gave corresponding 1,3,4-oxadiazoles [23].
R=4-H3CO-C6H4-, 4-OH-C6H4-, 4-Cl-C6H4, 2-thienyl
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Schem 8. Synthesis of pyrrolo [2,1-b]-1,3,4-oxadiazoles.
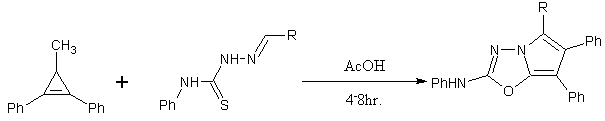
Direct synthesis of 1,3,4-oxadiazoles from carboxylic acid using cyanuric chloride/ indium under very mild conditions[24].
Scheme 9 Formation of 1,3,4-oxadiazoles from carboxylic acid using cyanuric chloride/indium
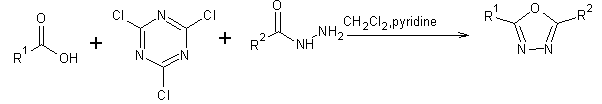
A series of novel 2-[1-(5-chloro-2-methyl-phenyl)-5-methyl-1H-pyrazol-4-yl]-5-(substituted –phenyl)-[1,3,4] oxadiazoles were synthesized from substituted benzoic acid[25].
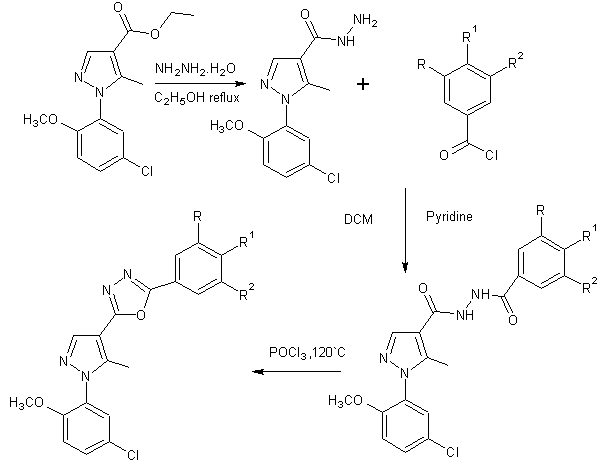
Scheme 10. Formation of 2-[1-(5-chloro-2-methoxy-phenyl)-5-methyl-1H-Pyrazol-4-yl]-5-(substituted-phenyl)-[1,3, 4]oxadiazoles.
The 2H-1-benzo/naphthopyran-2-one-4-yl(un)substituted phenyl-1,3,4-oxadiazoles has been synthesized by the oxidative cyclization of benzoic acid hydrazides formed in situ by the condensation of the respective 2H-1-benzo/naphthopyran-2-one-4-carboxaldehyde and (un)substituted monobenzoylhydrazide in moderate yield [26]
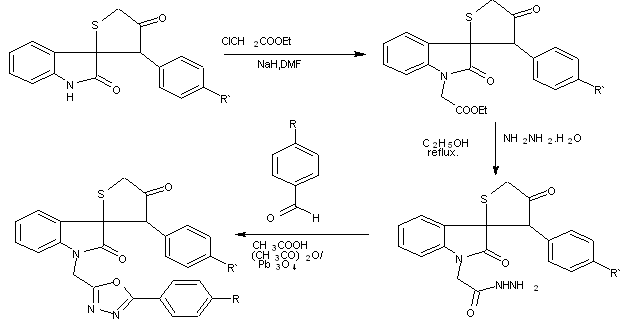
Scheme11 synthesis of 2-coumarinyl and spiroindolyl-5-phenyl-1,3,4-oxadiazoles.
A series of 1-(4-phenoxyphenyl)-3-[5-(substituted aryl)-1, 3,4-oxadiazol-2-yl]propan-1-one was synthesized by reaction of 3-(-phenoxybenzoyl)propionic acid with several hydrazides in phosphorus oxychloride[27].
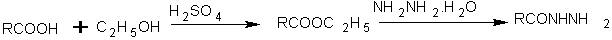
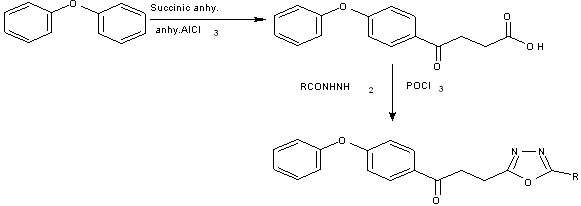
Scheme12 1-(4-phenoxyphenyl)-3-[5-(substituted aryl)-1,3,4-oxadiazol-2-yl]propan-1-one
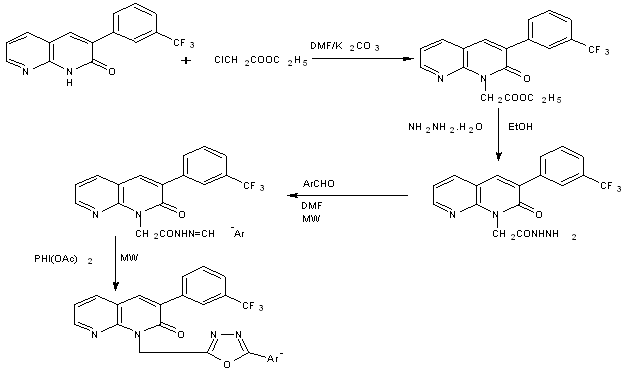
Scheme13 Synthesis of 1,3,4-oxadiazolyl 1,8-naphthyridines under microwave irradiation [28]
Oxidative cyclization of [o-(1,8-naphthapyridin-2-yl)phenoxy]acetic acid arylidene hydrazide with iodobenzene diacetate result in 5-aryl-2-[o-(1,8-naphthapyridin-2-yl)phenoxymethyl]1,3,4-oxadiazoles[29]
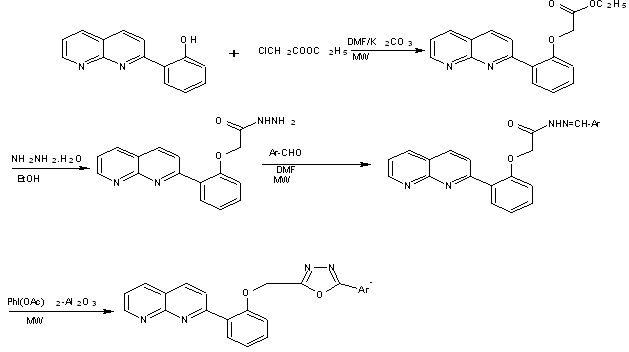
Scheme14 PhI(OAc)2-Al2O3 mediated synthesis of 1,8-naphthyridinyl-1,3,4-oxadiazoles under microwave irradiation
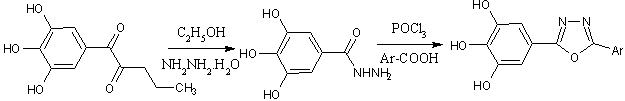
A series of 2-(3,4,5-trihydroxyphenyl)-5-aryl1,3,4-oxadiazoles were synthesized from propyl gallate and hydrazine hydrate in presence of ethanol to give 3,4,5-trihydroxybenzohydrazide followed by reaction with P0Cl3 and various aromatic acids[30].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Scheme15 synthesis of 2, 5-disubstituted 1,3, 4-oxadiazole derivative of Gallic acid.
Cyclodehydration of amino acid derived aryl hydrazide amide to the corresponding oxadiazole was followed by a 2nd dehydration event, smoothly furnishing the novel imidazo [5,1-b][1,3,4] oxadiazoles[31].
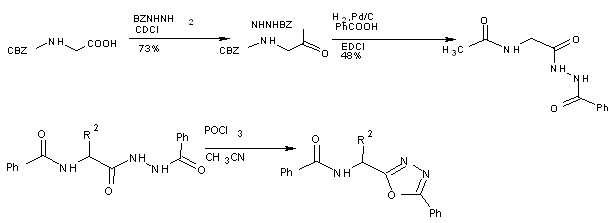
Scheme 16 Robust preparation of novel imidazo [5,1-b][1,3,4]oxadiazoles
One pot synthesis of 1,3,4-oxadiazoles from COOH and acylhydrazides was developed. Diacylhydrazide formation via. HATU coupling followed by addition of Burgess reagent afford the 1,3, 4-oxadiazoles[32].

Scheme 17 One-pot preparation of 1,3 ,4-oxadiazoles
One pot synthesis of 1, 3, 4-oxadiazole by diacylhydrazides via. Cyclization of diacylhydrazides using PPH3and hexachloroethane in presence of Huni`s base[33].
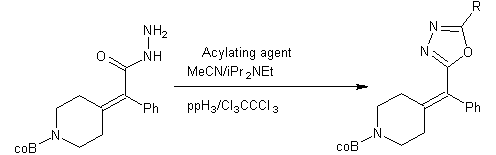
Scheme 18-general synthesis of tetrasubstituted alkenyl-1, 3, 4-oxadiazoles.
A series of disubstituted 1,3,4-oxadiazoles were prepared from Indole-2-carboxylic acid using thionyl chloride . The ring formation involve condensation reaction [34].
Scheme19 Synthesis of 1,3 ,4-oxadiazoles from indole2-carboxylic acid
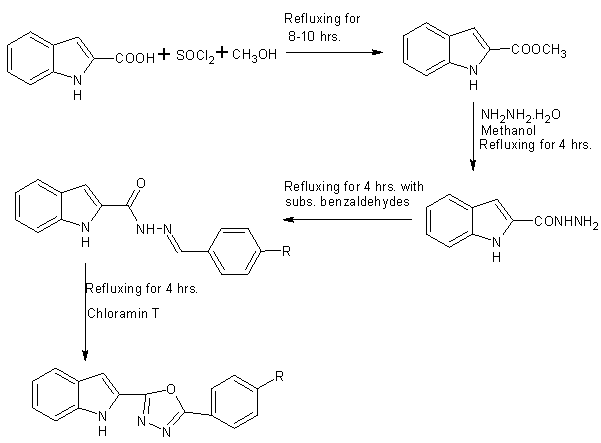
Scheme20 synthesis of some novel oxadiazoles of naphthol derivarives
2 amino-5-aryl-1, 3, 4-oxadiazoles were prepared from di(benzotriazolyl)methanimine[36].
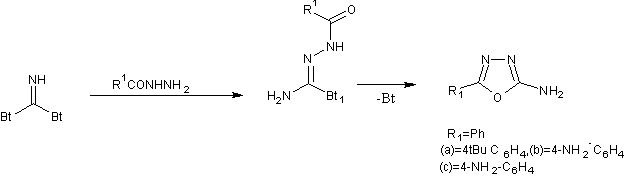
Scheme21-Snthesis of 2 amino-5-aryl-1,3, 4-oxadiazoles
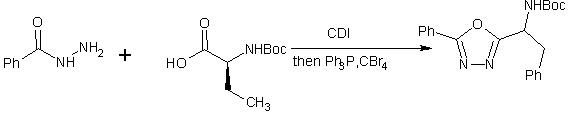
A one pot synthesis of 2,5-disubstituted 1,3,4-oxadiazole from acid and aryl hydrazide is reported ,acid activation with CDI followed by coupling with the desired acylhydrazide and dehydration in the same pot with Ph3P and CBr4 afford 1,3,4-oxadiazoles[37].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Scheme22. A mild and efficient one pot synthesis of 1,3,4-oxadiazoles from carboxylic acids and acyl hydrazides
Oxidation of a thiosemicarbazide result in formation of 2-amino1,3, 4-oxadiazole using 1,3-dibromo5-5dimethylhydantoin as oxidant[38].
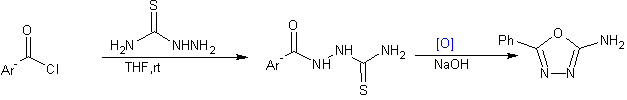
Scheme23 Synthesis of 2-amino-5-substituted -1,3, 4-oxadiazoles using 1,3-dibromo-5,5-dimethylhydantoin as oxidant:-
A series of 1,3,4-oxadiazoles were prepared from E-arylsulfpnylethenesulfonylacetic acid which is prepared from condensation of 1-arylsulfonyl2-chloroethene with mercaptopuric acid followed by oxidation and esterification[39].
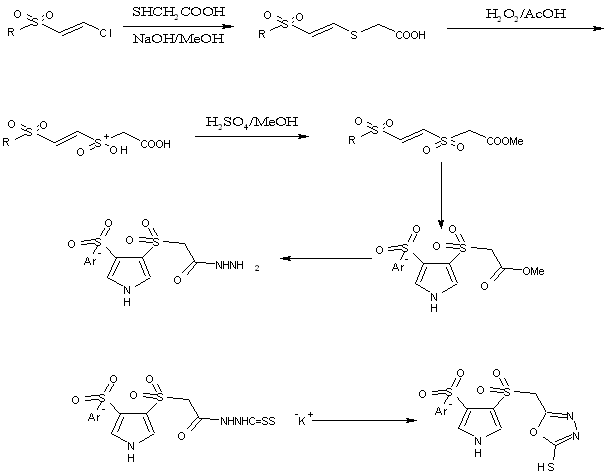
Scheme24. Preparation of new class of heterocycles pyrollyl oxadiazoles
A series of 2, 5-disubstituted 1, 3, 4-oxadiazoles were prepared from benzothiphene moiety. 3-chlorobenzo[b]thiophene-2-carboxylchloride was prepared from cinnamic acid and thionyl chloride in chlorobenzene medium using pyridine as catalyst and oxadiazoles were prepared from cyclization of hydrazides [40].
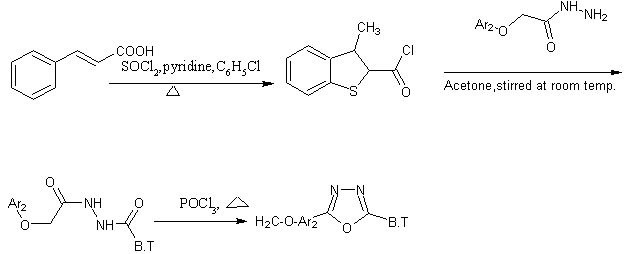
Schem25 Synthesis of some new benzo[b]thiophene as oxadiazoles
2-amino-5-(2-benzylthiophenyl)-1,3,4-oxadiazoles were prepared by reaction of thiosalicylic acid with appropriate benzyl chloride in alkaline hydromethanolic solution afford 2-benzylthiobenzoic acid, then esterification was done then hydrazides and hydrazides were converted to oxadiazoles [41].
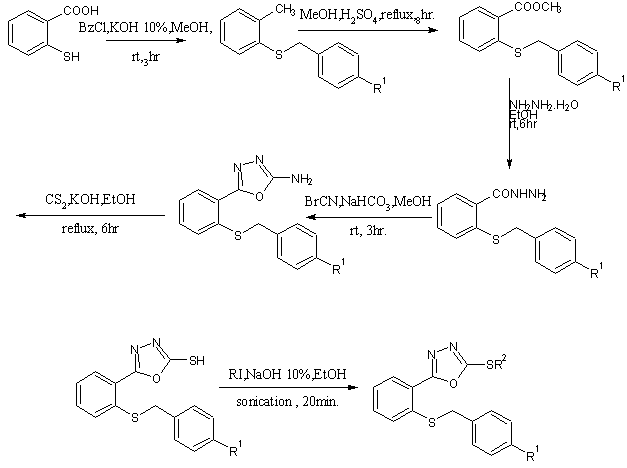
Scheme26. Synthesis of new 2-substituted -5-(2-benzylthiophenyl)-1,3, 4-oxadiazoles
Synthesis of peptidomimetic 2-arylamino-5-5ubstituted 1,3,4-oxadiazoles from BoC-protected α-amino acid derived hydrazides have been reported in a parallel solution phase synthesis[42].
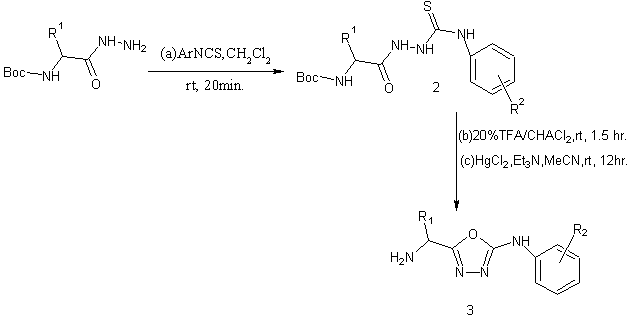
|
Entry |
Products |
R1 |
R2 |
|
1 |
2/3a |
Me |
H |
|
2 |
2/3b |
i-pr |
H |
|
3 |
2/3c |
Bn |
H |
|
4 |
2/3d |
Bn |
4-Me |
Scheme27.Parallel solution phase synthesis of a library of amino acid derived 2-arylamino-[1,3,4]-oxadiazoles.
Synthesis of 1,3,4-oxadiazoles by condensation of acid hydrazide and triethylortho alkanates under microwave irradiation using Nafion NR50 as solid support[43].
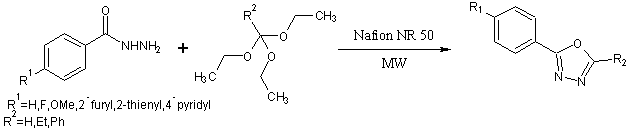
Scheme28.One pot solvent free synthesis of 1,3 ,4-oxadiazole
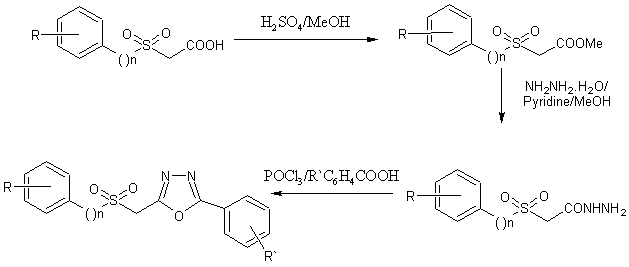
1,3 ,4-oxadiazoles were prepared from acid hydrazides on treatment with different carboxylic acid in presence of POCl3[44].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Scheme29. Synthesis of 1,3 ,4-oxadiazoles using acid hydrazides
Novel 1,3 ,4-oxadiazoles containing thienyl groups were synthesized from thiophene-2-carboxylic acid these acids can be transferred in to 1,3,4-oxadiazoles[45].
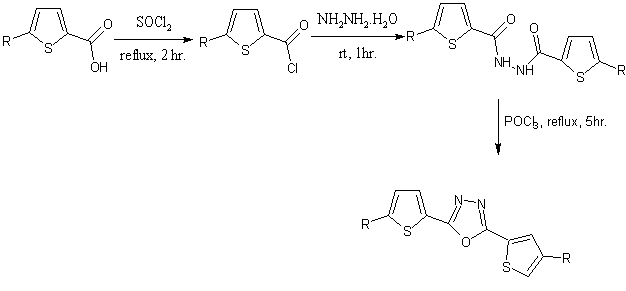
Scheme30.Novel analogue of α-Terthienyl 1,3,4-oxadiazole
4-amino benzoic acid was converted to 4-(toluene-4-sulfonylamino) benzoic acid by subjecting to p-toluene sulfonyl chloride which on treatment with hydrazine hydrate affords hydrazides then oxadiazoles were formed by reacting hydrazides with different aromatic aldehydes[46].
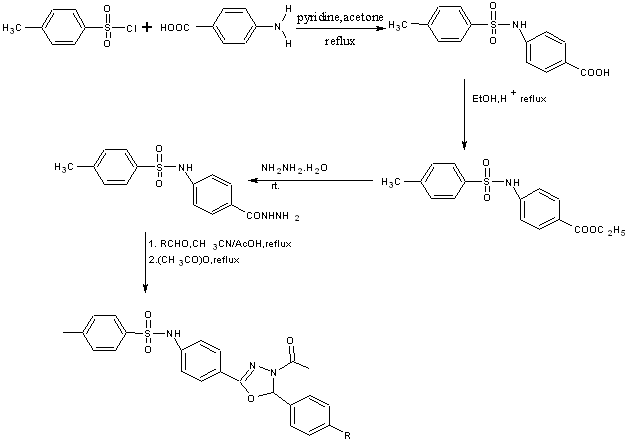
Scheme31. An efficient synthetic route for new 1,3,4-oxadiazoles having sulfonamide pharmacophore.
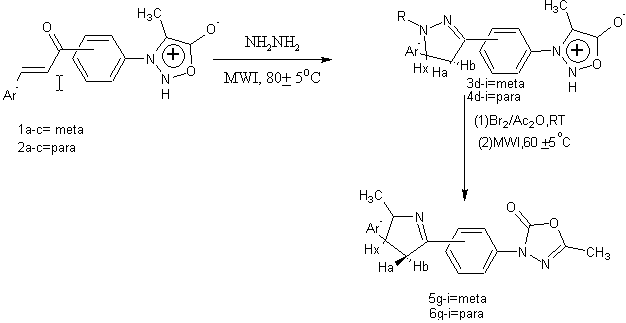
Scheme32. Expeditious synthesis of 1,3 4-oxadiazoles derivatives via. Syndnones[47]
2-[5-(aryl)-[1,3,4]oxadiazole-2-ylsulfanyl]alkanoic acid were synthesized from 2-[5-aryl)-[1,3,4]oxadiazole-2-ylsulfanyl] alkanoic acid[48].
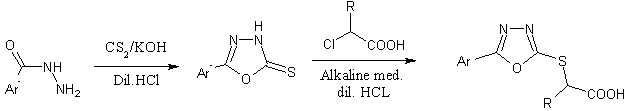
Ar = 4-Cl C6H4 R = CH3
Ar = 3-Cl C6H4 R = H
Ar = 2-Cl C6H4 R = CH3
Scheme33. Synthesis of some 2-[5-(aryl)-[1,3,4]oxadiazole-2-ylsulfanyl] alkanoic acid
2-mercaptoderivatives of 1,3,4-oxadiazoles can be prepared by reacting salicylic acid hydrazide with carbon disulfide followed by condensation reaction[49].
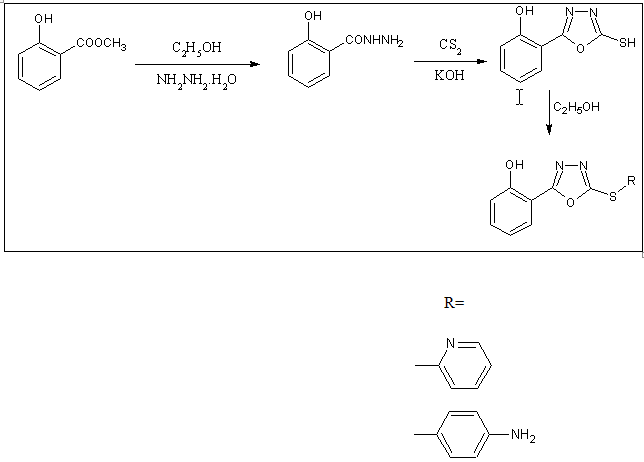
Scheme34. Synthesis of 2-mercapto-1,3,4-oxadiazoles
A series of per-o-benzoylated 5-(β-D-glucopyranosyl)-2-substituted-1,3,4-oxadiazoles were prepared by acylation of the corresponding 5-(β-D-glucopyranosyl)tetrazole[50].
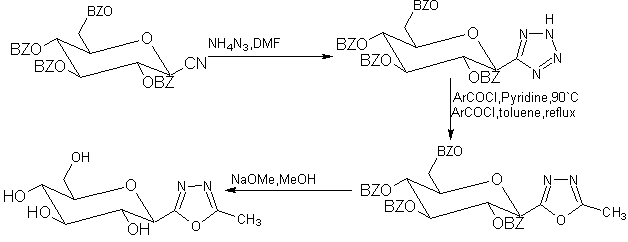
Scheme35 Synthesis of C-glycosylated oxadiazoles as inhibitor of glycogen phosphorylase.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2. Biological activity:-
2.1. Anticonvulsant Activity:-
Epilepsy is a common neurological disorder and a collective term given to agroup of syndromes that involve spontaneous, intermittent, abnormal electrical activity in the brain. The pharmacotherapy of epilepsy has been archieved during thelast decade. Furthermore, although for the lasttwenty years new antiepileptic drugs have been introduced into clinical practice, the maximal electroshock (MES) test and the subcutaneous pentylenetetrazole (scPTZ) test are the most widely used animal models of epilepsy to characterize the anticonvulsant activity. The heterocycles profound highly active in epilepsy one heterocycle is 1,3, 4-oxadiazole.
Mohammad et. al. (2009)synthesized a series of five membered heterocyclics in reaction between isoniazid and various substituted isothiocyanates and the compounds were tested for their anticonvulsant activity [51].
2-(4-chlorophenyl) amino-5-(4-pyridyl)-1, 3,4-oxadiazole
Bhat et al. (2008) synthesized a series of 3-(4 acetyl-5H/methyl-5-substituted phenyl-4, 5-dihydro-1,3,4-oxadiazol-2-yl)-2H-Chromen-2-ones. The synthesized compounds were evaluated for anticonvulsant activity [52].
1-[5-(2-methyl-2H-chromen-3-yl)-2-phenyl-1,3, 4-oxadiazol-3(2H)-yl]ethanone
Afshin et al. (2008)synthesized a new series of 2-substituted-5-{2-[(2-halobenzyl) thio)phenyl}-1,3,4-oxadiazoles and investigated for their anticonvulsant activity [53].
2-{2-[(2-fluorobenzyl)thio]phenyl}-5-(methylthio)-1,3,4-oxadiazole
Afshin et. al. (2005)synthesized a series of newly generated 2-substituted-5-(2- benzyloxyphenyl)-1,3,4-oxadiazoles and evaluated them for anticonvulsant activity[54
5-(2-benzyloxyphenyl)-2-mercapto-1,3,4-oxadiazole
Ali et al. (2004)synthesized a new class of 2-substituted-5-[2-(2-fluoro-phenoxy) phenyl]1,3,4-oxadiazoles derivatives and screened for their anticonvulsant activity [55].
2-methyl thio -5-[2-(2-fluorophenoxy)phenyl]-1,3,4-oxadiazole
2.2. Analgesic, Antiinflammatory and Antiplatelet Activity
Non-steroidal anti-inflammatory drugs (NSAIDs) have a wide clinical use for the treatment of inflammatory and painful conditions including rheumatoid arthiritis, soft tissue and oral cavity lesions, respiratory tract infections and fever. The two isoforms of cyclooxygenase (COX) are poorly distinguishable by most of the classical NSAIDs and these agents actually inhibit COX-1 extensively, besides COX-2, leading to gastrointestinal injury, suppression of TXA2 formation and platelet aggregation. The combination of these interactions is probably the reason for gastrointestinal bleeding asthe most serious complication of these drugs. 1,3,4-oxadiazole nucleus found satisfactory role in inflammatory and analgesic.
Akhtar et al. (2009) synthesized a series of 2,5-disubstituted-1,3,4-oxadiazoles which are aryolpropanoic acid derivatives. The synthesized compounds were evaluated for anti-inflammatory and analgesic activity [56].
3-[5-(5,8-dihydronaphthalen-1-ylmethyl)-1,3,4-oxadiazol-2-yl]-1-(4-methylphenyl)propan-1-one
Hussain et al. (2009)synthesized a novel series of 2-[3-(4-bromophenyl)propan-3-one]-5-(substituted phenyl)-1,3,4-oxadiazoles. The synthesized compounds showed good anti-inflammatory activity with reduced gastrointestinal side effects [57].
1-(4-methylphenyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl)propan-1-one
Hussain et al. (2008)synthesized a novel series of 1-(4-phenoxyphenyl)-3-[5-(substituted aryl)-1,3,4-oxadiazol-2-yl]propan-1-one. These compounds were tested for their anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation action [58].
1-(4-phenoxyphenyl)-3-(5-phenyl-1,3,4-oxadiazol-2-yl)propan-1-one
Rajak et al. (2007)synthesized naphthol derivatives of 1,3,4-oxadiazoles. The synthesized compounds showed good anti-inflammatory activity when compared to standard drug [59].
2-(4-chlorophenyl)-5-[(naphthalen-1-yloxy)methyL]-1,3,4-oxadiazole
Kadi et al. (2007)synthesized oxadiazoles of Adamantane 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles. The compounds showed good dose dependent anti-inflammatory activity [60].
2-(4-chlorophenyl)-5-(tricyclo[3.3.1.13,7]dec-1-yl)-1,3,4-oxadiazole
Ravindra et al. (2006)synthesized a series of 3-acetyl-5-naphthol[2,1-b]furan-2-yl-2-aryl-2,3-dihydro-1,3,4-oxadiazoles. The synthesized compounds showed very good anti-inflammatory activity [61].
1-[2-phenyl-5-(1,2,5,9b-tetrahydronaphtho[2,1-b]furan-2-yl)-1,3,4-oxadiazol-3(2H)-yl] ethanone
Khan et al. (2003)synthesized a series of 2-(coumarin-3-yl)-5-aryl-1,3,4-oxadiazole derivatives and these synthesized compounds were tested for their anti-inflammatory activity [62].
3-[5-(2-hydroxyphenyl)-1,3,4-oxadiazol-2-yl]-2H-chromen-2-one
Palaska et al. (2002)synthesized a series of 2-(2-naphthyloxymethyl)-5-substituted amino-1,3,4-oxadiazoles and evaluated them for anti-inflammatory activity [63].
2-(2-naphthyloxymethyl)5-aminophenyl-1,3,4-oxadiazole
Omar et al. (1996)synthesized a series of substituted 1,3,4-oxadiazole derivatives and the compounds were tested for anti-inflamatory activity [64].
N-cyclohexyl-5-(pyridin-3-yl)-1,3,4-oxadiazol-2-amine
2.3. Antimicrobial Activity
The dramatically rising prevalence of multi-drug resistant microbial infections in the past few decades has become a serious health care problem. The search for new antimicrobial agents will consequently always remain as an important and challenging task for medicinal chemists. Oxadiazoles found highly effective in bacterial and fungal diseases.
Farshori et al. (2010)synthesized a series of 5-alkenyl-2-phenylamine-1,3,4-oxadiazoles. The synthesized compounds were investigated for antibacterial and antifungal activities [65].
5-(dec-9-en-1-yl)-N-phenyl-1,3,4-oxadiazol-2-amine
Padmavathi et al. (2010)synthesized a series of pyrazol derivatives of 1,3,4-oxadiazole. Preliminary antimicrobial screening of the compounds showed that bis-heterocycle with a chloro substituted benzyl moiety shows high activity [66].
2-{[(4-chlorobenzyl)sulfonyl]methyl}-5-[(4R)-4-(4-chlorophenyl)-4,5-dihydro-1H-pyrazol-3-yl]-1,3,4-oxadiazole
Xu et al. (2009)synthesized 2,5-disubstituted-1,3,4-oxadiazoles containing azulene moiety. The synthesized compounds showed very good antifungal activity [67].
2-(3-methylazulen-1-yl)-5-phenyl-1,3,4-oxadiazole
Padmavathi et al. (2009)synthesized a series of heterocycles 2-(arylsulphonylmethyl)-5-aryl-1,3,4-oxadiazoles. These compounds showed antimicrobial activity against the Gram +ve bacteria Staphylococcus Aureus, Bacillus Subtilis and the Gram –ve bacteria Klebsiella Pneumonia etc. [68].
2-[(benzylsulfonyl)methyl]-5-phenyl-1,3,4-oxadiazole
Rai et al. (2009)synthesized a series of 2-[1-(5-chloro-2-
methoxy-phenyl)-5-methyl-1H-pyrazol-4-yl]-5-(substituted-phenyl)-[1,3,4]-oxadiazoles. The synthesized compounds were found active against Bacillus subtilis, Staphylococcus aureus, E.coli respectively, when compared with standard drug ampicillin [69].
2-[3-(5-chloro-2-methoxyphenyl)cyclopenta-1,4-dien-1-yl]-5-(4-chlorophenyl)-1,3,4-oxadiazole.
Chandrakantha et al. (2009)synthesized a series of 1,3,4-oxadiazole derivatives containing 2-fluoro-4-methoxy moiety. All the newly synthesized compounds were screened for their antibacterial and antifungal activity [70].
2-(3-bromo-2-methylphenyl)-5-(2-fluoro-4-methoxyphenyl)-1,3,4-oxadiazole
Jain et al. (2009)synthesized a series of 2-[5-(aryl)-[1,3,4]oxadiazole-2-ylsulfanyl]alkanoic acid. The synthesized compounds were evaluated for antimicrobial activity against Gram positive and Gram negative bacterial strains [71]
2-{[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}propanoic acid
Saini et al.(2009)synthesized a series of 5-(benztriazole-1-yl-methyl)-2-substituted 1,3,4-oxadiazoles. Thes synthesized compounds were evaluated for their antibacterial activity [72].
4-[5-(1H-benzotriazol-1-ylmethyl)-1,3,4-oxadiazol-2-yl]aniline
Karthikeyan et al. (2008) synthesized analogue of 2,5-disubstituted-1,3,4- oxadiazoles which shows antimicrobial activity against S. aureus & P. aeruginosa and Antifungal activity against Candida Albicans, Aspergillus Fumigatus, Penicillum Marneffei [73].
2-(2,4-dichloro-5-fluorophenyl)-4-{5-[(4-methylphenoxy)methyl]-1,3,4-oxadiazol-2-yl}quinoline
Gupta et al. (2008)synthesized 3-[5-(4-substituted) phenyl-1,3,4-oxadiazole-2yl]-2-styrylquinazoline-4(3H)-one. The synthesized compounds were evaluated for their antibacterial activity against staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and E. coli etc. [74].
3-[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl]-2,4-dimethyl-3,4-dihydroquinazoline
Gaonkar et al. (2006)synthesized a series of novel 2-{4-[-2-(5-ethylpyridin)-2yl ethoxy] phenyl}-5-substituted -1,3,4-oxadiazoles. The synthesized compounds showed good antibacterial activity, when compare with standard drug [75].
2-(2-{4-[5-(2,3-dichlorophenyl)[1,3,4-oxadiazol-2yl]-phenoxy}ethyl)-5-ethylpyridine.
Shaker et al. (2005)synthesized bis-1,3,4-oxadiazoles and the synthesized compounds showed very good anti bacterial and antifungal activity [76].
5,5'-benzene-1,4-diylbis(N-phenyl-1,3,4-oxadiazol-2-amine)
Vosooghi et al. (2005)synthesized a series of 2-(4-nitro-1-imidazolylmethyl)-1,3,4-oxadiazoles. The synthesized compounds were evaluated for their antimicrobial activity against Bacillus Subtilis, Clostridium difficile, Aspergillus niger and Cryptococcus neoformans [77].
5-[(2-methyl-4-nitro-1H-imidazol-1-yl) methyl]-1,3,4-oxadiazol-2-amine
Emam et al. (2004)synthesized analogues of 5-(1-adamantyl)2-ethyl or substituted ethylthio-1,3,4-oxadiazoles. The synthesized compounds showed good activity against various microbes such as gram positive and gram negative bacteria [78].
2-(ethylsulfanyl)-5-(tricyclo[3.3.1.13,7]dec-1-yl)-1,3,4-oxadiazole
Kocabalkanli et al. (2001)synthesized a series of 2-[[α-(4-substitutedbenzoyloxy)-α-phenylacetyl]amino]-5-(4-methoxyphenyl)-1,3,4-oxadiazoles. These novel compounds were evaluated for their antibacterial activity against various bacterial and fungal strains [79].
1-{[5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl]amino}-1-oxopropan-2-yl benzoate
Ates et al. (1998)synthesized a series of 5-aryl-2-[(N,N-disubstituted thiocarbamoylthio)acylamino]-1,3,4-oxadiazoles. The synthesized compounds were tested against various microorganisms such as staphylococcus aureus and staphylococcus epidermidis [80].
1-oxo-1-[(5-phenyl-1,3,4-oxadiazol-2-yl)amino]propan-2-yl dimethylcarbamodithioate
2.4. Antitubercular activity
Tuberculosis is a serious health problem that causes the death of some three million people every year worldwide. In addition to this, the increase in Mycobacteriumtuberculosis strains resistant to front-line antimycobacterial drugs such as rifampin and isoniazid has further complicated the problem, which clearly indicates the need for more effective drugs for the efficient management of tuberculosis.
Siddiqui et al. (2010)Synthesized a series of 5-{3`-oxo-6`-(substituted aryl)-2`,3`,4`,5`-tetrahydropyridazin-2`ylmethyl}-2-substituted-1,3,4-oxadiazoles. The synthesized compounds were evaluated for their antitubercular activity [81].
6-phenyl-2-{[5-(phenylamino)-1,3,4-oxadiazol-2-yl]methyl}-4,5-dihydropyridazin- 3(2H)- one
Pattan et al. (2009)synthesized a series of 5-(2-hydroxyphenyl)-2-phenyl-1,3,4-oxadiazole and screened for biological activity. The synthesized compounds showed good antitubercular activity, when compared to standard drug [82].
2-{5-[(4-aminophenyl) sulfanyl]-1,3,4-oxadiazol-2-yl}phenol
Joshi et al. (2008)synthesized a series of 5-substituted-2-thiol-1,3,4-oxadiazole derivatives. The synthesized compounds were screened for antitubercular activity against mycobacterium tuberculosis H37Rv strains [83].
1-{5-phenyl-2-[4-(pyrrolidin-1-yl)phenyl]-1,3,4-oxazol-4-yl}ethanone
Vazquez et al. (2007)synthesized a series of 4-(5-substituted-1,3,4-oxadiazol-2-yl)pyridine derivatives and evaluated them for antimicrobial activity. MIC values for these were 0.35 µm against H37 Rv [84].
4-(5-pentadecyl-1,3,4-oxadiazol-2-yl)pyridine
Ali et al. (2007)synthesized a series of 3-{2-furfuryl[4-(4-{2-furyl[5-(2-naphthyloxymethyl)-2-thioxo-2,3-dihydro-1,3,4-oxadiazol-3—phenylsulfonyl)aniline] methyl}-5-(2-naphthyloxymethyl)-2,3-dihydro-1,3,4-oxadiazoles-2-thione.The synthesized compounds showed very good activity against the growth of mycobacteria with MIC 0.1µm [85].
3-{2-furfuryl[4-(4-{2-furyl[5-(2-naphthyloxymethyl)-2-thioxo-2,3-dihydro-1,3,4-oxa- diazol-3-yl]methylamino}phenylsulfonyl)aniline]methyl}-5-(2-naphthyl oxy methyl)-2,3-dihydro-1,3,4-oxadiazoles-2-thione.
Yar et al. (2007)synthesized 2,5-disubstituted-1,3,4-oxadiazoles. The synthesized comounds showed very good antimycobacterial activity against a strain of mycobacterium tuberculosis [86].
N-(4-chlorophenyl)-5-phenyl-1,3,4-oxadiazol-2-amine
Macaev et al. (2005)synthesized a series of 5-aryl-2-thio-1,3,4-oxadiazoles. The synthesized derivatives showed good inhibitory activity against Mycobacteria when compared to standard drug isoniazid [87].
4-phenyl-5-[(5-phenyl-1,3,4-oxadiazol-2-yl)sulfanyl]-1,3-thiazol-2-amine
Foks et al. (2004)synthesized (4-phenylpiperazin-1-ylmethyl)-1,3,4-oxdiazole. The compounds obtained were tested in vitro for their tuberculostatic activity. The minimum inhibiting concentration was determined and were found between 25-100 ?g/ml [88].
1-[(5-ethoxy-1,3,4-oxadiazol-2-yl)methyl]-4-phenylpiperazine
Kucukguzel et al. (2002)Synthesized a series of 2-[4-(4-methoxybenzoylamino) phenyl]-5-(substituted phenyl) amino-1, 3, 4-oxadiazoles 1, 3, 4-oxadiazoles. The compounds showed good bacteriostatic effect against E.coli and Enterobacter aerogens [89].
4-methoxy-N-(4-{5-[(4-methylphenyl) amino]-1, 3, 4-oxadiazol-2-yl}phenyl)benzamide
2.5. Antimitotic Activity
A variety of antitumoral drugs are currently in clinical use. The search for antitumoral drugs led to the discovery of several 1,3,4-oxadiazoles having antitumoral activity.
Kiselyov et al.(2010)synthesized a series of 2,5-disubstituted-1,3,4-oxadiazoles. The synthesized compounds were analysed by using the sea urchin embryo test system. The synthesized compounds showed as good as activity as podophyllotoxin [90].
N-[(3-{5-[(4-chlorophenyl)amino]-1,3,4-oxadiazol-2-yl}-1,2-thiazol-4-ylmethyl] pyridin-3-amine
Dalip et al.(2009)synthesized a series of novel 5-(3-indolyl)-2-(substituted)-1,3,4-oxadiazoles. The oxadiazoles were screened for their in vitro anticancer activity against various human cancer cell lines [91].
3-[5-(pyridin-4-yl)-1,3,4-oxadiazol-2-yl]-1H-indole
Muragila et al. (2008)synthesized a new series of 2-trifluoroacetylthiophene oxadiazoles. These oxadiazoles are used in cancer therapy as histone deacetylase inhibitors [92].
2,2,2-trifluoro-1-[5-(5-phenyl-1,3,4-oxadiazol-2-yl)thiophen-2-yl]ethanone
2.6. Hypoglycaemic activity:-
Toth et al. (2009)synthesized a series of 5β-D-glucopyranosyl-2-substituted-1,3,4-oxadiazoles. The formed oxadiazoles showed weak inhibitory activity of glycogen phosphorylase [93].
2-(hydroxymethyl)-6-(5-methyl-1,3,4-oxadiazol-2-yl)tetrahydro-2H-pyran-3,4,5-triol
2.7. Miscellaneous activities:-
2.7.1. Insecticidal Activities:-
Milinkevich et al. (2009)synthesized a series of 2-(4,5-dihydroisoxazol-5-yl)-1,3,4-oxadiazoles. Several compounds of this series were found active against fungal and pest insects [94].
2-(4-chlorophenyl)-5-[5-methyl-3-(4-nitrophenyl)-4,5-dihydro-1,2-oxazol-5-yl]-1,3,4-oxadiazole
Liu et al. (2004)synthesized a series of thienyl 1,3,4-oxadiazoles. The synthesized compounds were evaluated for southern armyworm for their insecticidal activity [95].
2-(5-nitrothiophen-2-yl)-5-(thiophen-2-yl)-1,3,4-oxadiazole
Zheng et al. (2003)Synthesized a series of novel 2,5-disubstituted-1,3,4-oxadiazoles. The synthesized compounds showed significant inhibitory activity against army worms [96].
2-(2,4-dichloro-5-fluorophenyl)-5-(2,3,4,5-tetrafluorophenyl)-1,3,4-oxadiazole
Cao et al. (2002) synthesized 2-(5-trifluoromethyl) pyridyloxymethyl)-1,3,4-oxadiazoles. The preliminary bioassay tests showed that some compounds especially those having fluorine on the benzene ring exhibited a significant insecticidal activity on armyworm at 500mg/L [97].
2-phenyl-5-{[3-(trifluoromethyl)phenoxy]methyl}-1,3,4-oxadiazole
Shi et al. (2000)synthesized a series of 2-fluorophenyl-5-substituted cyclopropyl-1,3,4-oxadiazoles with cis/trans isomers and the synthesized compounds showed insecticidal activity against armyworms [98].
2-(2,4-dichloro-5-fluorophenyl)-5-[3-(dichloromethylidene)-2,2-dimethylcyclopropyl]-1,3,4-oxadiazole
Shaban et al. (1991)synthesized a series of 3-acetyl-2,5-diaryl-2,3-dihydro-1,3,4-oxadiazoles. The synthesized compounds were evaluated for nematocidal, insecticidal, and herbicidal activities [99].
2,5-bis(4-chlorophenyl)-3-phenyl-2,3-dihydro-1,3,4-oxadiazole
2.7.2. Vasorelaxant Effects
Relaxation of vascular smooth muscles is a good treatment strategy in hypertension, angina and other cardiovascular disorders. Oxadiazoles have been reported to have effect on vascular smooth muscles and calcium influx.
Bankar et al. (2009)synthesized a series of 4-[3-acetyl-5-(pyridine-3yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl]phenyl acetate. The synthesized compounds were evaluated for their vasorelaxant effect [100].
4-[3-acetyl-5-(pyridin-3-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl]phenyl acetate
2.7.3. Diagnostic agents
Watanabe et al. (2009)synthesized a series of radioiodinated 2,5-diphenyl-1,3,4-oxadiazoles. The synthesized compounds were used for detecting β-amyloid plaques in Alzheimer`s brains [101].
2-(4-iodophenyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole
2.7.4. Antiviral Activity
HIV infection and AIDS represent one of the first diseases for which the discovery of drugs was performed entirely via a rational drug design approach. Current treatment regimens are based on the use of two or more drugs that belong to group of inhibitors termed as highly active antiretroviral therapy (HAART).
Tan et al. (2006)synthesized a series of 2-benzenesulfonyl-5-substituted-sulfanyl-[1,3,4]-oxadiazoles. The synthesized compounds were having very good activity against hepatitis virus [102].
1-{2-[5-(1-benzenesulfonyl-propyl)-[1,3, 4]oxadiazol-2-yl-sulfanyl]-ethyl}-4-(2-methoxy-phenyl)-piperazine.
Emam et al. (2004)synthesized a series of 5-(1-Adamantyl)-2-substituted thio-1,3,4-oxadiazoles. The synthesized compounds were tested using the XTT assay method. The compounds showed high reduction of viral replication [103].
5-(tricyclo[3.3.1.13,7]dec-1-yl)-1,3,4-oxadiazole-2(3H)-thione
FIND ALL ABOVE MENTIONED STRUCTURES
References:
1. Somani R. R, Shirodkar P. Y. Der pharma Chemica 1 (2009) 130-140.
2. Dabiri M, Salehi P, Baghbanzadeh M, Bahramnejad M. Tetrahedron Letters 47 (2006) 6983-6986.
3. Leung D, Du W, Hardouin C, Cheng H, Hwang I, Cravatt B.F, Boger D. L. Biorg. Med. Chem. Lett.15 (2005) 1423.
4. Guimaraes C R, Boger D L, Jorgensen W L. J. Am. Chem. Soc. 127 (2005) 17377-17384.
5. Borg S, Vollingra R. C, Labarre M, Payza K, Terenius L, Luthman K. J. Med. Chem.42 (1999) 4331-4342.
6. Polshettiwar V, Varma R. S. Tetrahedron Letters 49 (2008) 879-883.
7. Hays F N, Rogers B S, Off D G. J. Am. Chem. Soc.77 (1955) 1850-1852.
8. rown A R, Bradley D D C, Burns P L, Burroughes J H, Friend R H, Greenham N C, Burn P L. Appl. Phys. Lett. 61 (1992) 2799-2795.
9. Steigbigel R. T, Cooper D. A, Kumar P. N. Engl. J. Med. 359 (4) 339–54.
10. Ogata M, Atobe H, Kushida H, Yamamoto K. J. Antibiot. 24 (1971) 443-451.
11. Vardan S, Mookherjee S, Eich R. Clin. Pharm. Ther. 34 (1983) 290-296.
12. Schlecker R, Thieme P. C. Tetrahedron 44 (1988) 3289-3294.
13. Dehaen W, T. H. Ngo. Comprehensive Heterocyclic Chem. 3 (2008) 427-446.
14. Rostamizadeh S, Ghasem S.A. Tetrahedron Letters 45(2004) 8753-8756
15. Zarudnitskii E.V, Pervak I, Merkulov A.S, Yurchenko A.A, Tolmachev A.A.
16. Tetrahedron 64 (2008)0431-10442
17.Kangani C.O, Kelley D.E, Day B.W, Tetrahedron Letters 47(2006) 6497.
18. Milinkevich K. A, Yoo C.L, Sparks T.C, Lorsbach B.A, Kurth M.J. Bioorg. Med. Chem. Lett. 19 (2009) 5796-5798.
19. Dolman S.J, Gosselin F, Davies I.W. J. Org. Chem. 71 (2006) 9548
20. Dobrota C, Paraschivescu C, Dumitro L, Matache M, Baciu I, Ruta L.L, Tetrahedron Letters 50 (2009) 1886-1888
21. Wang Y, Sauer D.R, Djuuric S.W. Tatrahedron Letters 47 (2006) 105-108
22. Tsoleridis C.A, Stephanidou J.S, Gounaridis P, Zika H, Pozarentzi M. Tetrahedron Letters 59 (2003) 4591-4601.
23. Wang L.N, Han D, Xu F.F, Meng X.B, Li Z.J. Carbohydrate Research 344 (2009) 2113-2119.
24. Ali A.A, Hassan A.A, Ameen M.A, Brown A.B. Tetrahedron Letters 49 (2008) 4060-4062
25. Kangani C.O, Day B.W. Tetrahedron Letters 50 (2009) 5332-5335.
26. Rai N. P, Narayanaswamy V. K, Shashikanth S, Arunachalam P. N. Europeon J. Med. Chem. 44 (2009) 4522-4527.
27. Kenny R.S, Mashelkar U.C, Rane D.M. J. Chienese Chem. Soc. 53 (2006) 367-373.
28. Hussain A, Ahmad F.J, Ajmal M, Ahuja P. J. Serb. Chem. Soc.73 (2008) 781-791.
29. Mogilaiah K, Babu H.S, Prasad R.S. Ind. J. Chem. 48 (2009) 868-872.
30. Mogilah K, Anita E, Babu S, Swamy T.K. J. Het. Chem. 46 (2009)127.
31. Arunkumar S, K.Llango, Manikandan R.S, Sudha M, Ramalakshmi N. International J. Chem. Tech. Res.1 (2009) 1094-1099
32. Tuan P, Patel N, Samas B, Schwarz B.Org. Biomol. Chem. 7 (2009) 5063-5066
33. Changkun Li, Hamilton D. Tetrahedron Letters 50 (2009) 6435-6439
34. James C.A, Poirier B, Grise G, Martel A, Ruediger E.H. Tetrahedron Letters 47 (2006) 511-514
35. Bhardwaz N, Saraf S.K, Sharma P, Kumar P. Eur. J. Chem. 6(2009) 1133-1138
36. Rajak H, Kharya M.D, Mishra P. The Pharmaceutical Society of Japan 127 (2007) 1764
37. Katritzky A.R, Vvedensky V, Cai X, Rogovoy B, Steel P.J. ARKIVOC 6 (2002) 82-90
38. Rajapakse H.A, Zhu H, Young M.B, Mott B.T. Tetrahedron Letters 47 (2006) 4827-4830
39. Rivera N.R, Balsells J, Hansen K.B. Tetrahedron Letters 47 (2006) 4889-4891
40. Padmavathi V, Nagendra A.V, Thiveni P, Shazia A. Eur. J. Med. Chem. 44 (2009) 2313-2321
41. Isloor A.M, Kalluraya B, Pai K.S. Eur. J. Med. Chem. 30 (2009) 1-6
42. Zarghi A, Faizi M, Shafeghi B, Ahadian A, Khojastephpoor H.R, Zanganeh V, Tabatabi S.A, Shaffire A. Bioorg. Med. Chem. Lett. 15 (2005) 3126-3139
43. Gavrilyuk J.I, Lough A.J, Batey R.A. Tetrahedron Letters 49 (2008) 4746-4749
44. Polshettiwar V, Verma R.S. Tetrahedron Letters 49 (2008) 879-883
45. Padmavathi V, Reddy G.S, Padmaja A, Kondaiah P, Shazia A. Eur. J. Med. Chem. 44 (2009) 2106-2112
46. Liu L.G, Xu Y.F, Qian X.H, Huang Q.C. Chienese Chem. Lett. 15 (2004) 7-10
47. Jagrut V.B, Netankar P.D, Jawale D.V, Mane R.A, Jadhav W.N. Bull Korean Chem. Soc. 30 (2009) 2812-2814
48. Kamble R.R, Sudha B.S, Bhadregowda D.G.J. Serb. Chem. Soc.73 (2008) 131-138
49. Jain N, Pathak D.P, Mishra P, Jain S. J. Iran. Chem. Soc. 6 (2009) 77-81
50. Pattan S.R, Rabara P.A, Pattan J.S, Bukitagar A.A, Wakale V.S, Musmade D.S. Indian J. Chem. 48 (2009) 1453-1456
51. Toth M, Kun S, Bokor E, Benltifa M, Tallec G, Vidal S, Docsa T, Somsak L, Praly J.P. Biorg. Med. Chem. Lett. 17 (2009) 4773-4785
52. Yar M. S, Akhtar M. W. Acta Poloniae Pharmaceutica-Drug Research, 66 (2009) 393-397.
53. Mashooq A. Bhat, Siddiqui N and Khan S. A. Acta poloniae pharmaceutica, 65 (2008) 235-239.
54. Zargahi A, Hamed S, Tootooni F, Amini B, Sharifi B, Faizi M, Abbas S. Sci Pharm.76 (2008) 185-201.
55. Zarghi A, Faizi M, Shafaghi B, Ahadian A, Hamid R. Khojastehpoor, V. Zanganeh, Sayyed A. Tabatabai and Abbas Shafiee. Bioorg. and Med. Chem. Lett. 15 (2005) 3126-3129.
56. Almarisad A, Tabatabi S. A, Faizi M, Kebriaeezadeh A, Mehrabi N, Dalvandi A. Bioorg. & Med. Chem. Lett. 14 (2004) 6057-6059.
57. Akhtar M, Hussain A, Bismillah Azad, Ajmal M. Eur. J. Med. Chem. 44 (2009) 2372-278.
58. Hussain A, Ajmal M. Acta Pharm. 59 (2009) 223-233.
59. Hussain A, Ahmad F. J, Ajmal M, Ahuja P. J. Serb. Chem. Soc. 73 (2008) 781-791.
60. Rajak H, Kharya M. D, Mishra P. The pharmaceutical society of Japan 127 (2007) 1757-1764.
61. Adan A. Khdi, Nasser R. El-Brollosy, Omar A. Al-Deeb, Elsayad E.Habib, Tarek M. Ibrahim. Eur. J. Med. Chem. 42 (2007) 235-242.
62. K. C. Ravindra, H. M. Vagdevi, V. P. Vaidya, B. Padmashali. Ind. J. Chem. 45 (2006) 2506-2511.
63. Khan M. S. Y, Akhtar M. Ind. J. Chem. 42 (2003) 900-904.
64. Palaska E, Sahin G, Kelicen P, Tugba Dulru. IL Farmaco 57 (2002) 101-107.
65. Omar F. A, Mahfouz N. M, Rahman M. A. Eur. J. Med. Chem. 31 (1996) 819-825.
66. Nida N. Farshori, Mudasir R. Banday, Anis Ahmad, Asad U. Khan, Abdul Rauf. Bioorg. & med. chem. Lett. 20 (2010) 1933-1938.
67. Padmavathi V, Reddy G. S, Raddy G. D, Payani T. Synthetic communication 40 (2010) 482-493.
68. XU Jiao, Dao-Lin Wang, Kimiaki Imafuku. Synthetic communication 39 (2009) 2196-2204.
69. Padmavathi V, Sudhakar G Reddy, Padmaja A, Kondaiah P, Shazia A. Eur. J. Med. Chem. 44 (2009) 2106-2112.
70. Premsai N Rai, Narayanaswamy V. K, Sheena Shashikanth, Arunachalam P. N, Eur. J. Med. Chem. 44 (2009) 4522-4527.
71. Chandrakantha B, Shetty P, Nambiyar V, Nishita Isloor, Isloor A.M. Eur. J. Med. Chem. 30 (2009) 1-5.
72. Jain N, Pathak D. P, Mishra P, Jain S. J. of Iran Chemical Society 6 (2009) 77-81.
73. Saini R, Rai A. K, Ansari A. N, Shahar M Yar. Asian J. of Chemical Research 21 (2009) 34-36.
74. Karthikeyen M. S, Prasad D. J, Mahalinga M, Bantwal S. Holla, Kumari N. S. Eur. J. Med. Chem. 43 (2008) 25-31.
75. Gupta V, Kashaw S. K, Jatav V, Mishra P. Med. Chem. Res, 17 (2008) 205-211.
76. Ganokar S. L,Rai K. M. L, Prabhuswamy B. Eur. J. Med. Chem. 41 (2006) 841-846.
77. Shaker R. M, Mahmoud A. F, Abdel Latif F. F. Phosphorus Sulphur and Silicon. 180 (2005) 397-406.
78. Vasooghi M, Akbarzadeh T, Fallah A, Fazeli M. R, Jamalifar H, Shafiee A. J. of science, Islamic republic of Iran 16 (2005) 145-151.
79. Ali A. El-Emam, Omar A. Al-Deeb, M. Al-Omar, J. Lehmann. Bioorg. & Med. chem. 12 (2004) 5107-5113.
80. Kocabalkanli A, Ates O, Otuk G. IL Farmaco 56 (2001) 975-979.
81. Ates O, Kocabalkanli A, Cesur N, Otuk G. IL Farmaco 53 (1998) 541-544.
82. Siddiqui A, Islam M, Kumar S. Der Pharmacia Lettre. 2 (2010) 319-327.
83. Pattan S. R, Rabara P.A, Pattan J. S, Bukitagar A. A, Wakale V. S, Musmade D. S. Ind. J. Chem. 48 (2009) 1453-1456.
84. Joshi S. D, Vagdehi H. M, Eur. J. Med. Chem. 43 (2008) 1989-1996.
85. Vazquez G. N, Salinas G. M. M, Fajardo Z. V. D, Villareal J. V, Soto S. E, Salazar F. G, Nunez E. H. Bioorg. & Med. Chem. Lett.15 (2007) 5502-5508.
86. Ashraf Ali M, Shaharyar M. Biorg. & Med. Chem. Lett. 17 (2007) 3314-3316.
87. Yar M, Siddaqui A. A, Ashraf Ali M. J. Chienese Chem. Soc. 54 (2007) 5-8.
88. Macaev F, Rusu G, Pogrebnoi S, Gudima A, Stingaci E, Vlad L, Shvets N, Kandemirli F, Anatholy Dimoglo, Reynolds R. Biorg. & med. chem.13 (2005) 4842-4850.
89. Foks H, Pancechowska D, Janowiec M, Zwolska.Z, Kopec E. A. Acta Poloniae pharmaceutica 61 (2004) 473-476.
90. Kucukguzel S. G, Oruc E. E, Rollas S, Sahin F, Ozbek A. Eur. J. Med. Chem. 37 (2002) 197-206.
91. Kiselyou A. S, Semenova M. N, Chernyshova N. B, Leitao A, Samet A. V, Kislyi K. A, Raihstat M. M, Opera T, Lemcke H, Lantow M. Eur. J. Med. Chem. 45 (2010) 1683-1697.
92. Kumar D, Sundaree S, Johnson E. O, Shah K. Bioorg. & Med. Chem. Lett. 19 (2009) 4492-4494.
93. Murglia E, Altamura S, Branca D, Cecchetti O, Ferrigno F, Vittoria Orsale M, Palumbi M. C, Rowley M, Scarpelli R. Bioorg. & Med. chem. Lett.18 (2008) 6083-6087.
94. Toth M, Kun S, Bokor E, Benltifa M, Tallec G, Vidal S, Docsa T. Bioorg. & Med. Chem.17 (2009) 4773-4785.
95. Milinkevich K.A, Choong L.Yoo, Thomas C. Sparks, Beth A. Lorsbach. Biorg. Med. Chem. Lett. 19 (2009) 5796-5798.
96. Li Gang LIU, YU Fang XU, XU Hong QIAN. Chienese Chemical Letters 15 (2004) 7-10.
97. Zheng X, Zhing Li, Wang Y, Chen W, Huang Q, Liu C, Song G. J. Fluorine Chem. 123 (2003) 163-169.
98. Cao S, Qian X, Song G, Huang Q. J. Fluorine Chem. 117 (2002) 63-66.
99. Shi W, Qian X, Song G, Zhang R, Li R. J. Fluorine Chem. 106 (2000) 173-179.
100. Shaban M. A. E, Nasar A. Z, Badry S. M. Journal of Islamic Academy of Science 4:3 (1991) 184-191.
101. Bankr G. R, Nandakumar K, Nayak P. G, Thakur A, Rao M Chamallamundi, Nampurth G. P. Chemico-Biological Interaction 181 (2009) 377-382
102. Watanabe H, Ono M, Lkeoka R, Haratake M, Saji H, Nakayama M. Bioorg. & med. chem. 17 (2009) 6402-6406.
103. Tan T. M. C, Chen Yu, Kong K. H, Bai J, Li Y, Lim S. G, Ang T. H, Lam Y. Antiviral Research 71 (2006) 7-14.
104. Emam A. A, Omar A, Al-Omar M, Lechmann. J. Bioorg. Med. Chem. 12 (2004) 5107-5113.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









