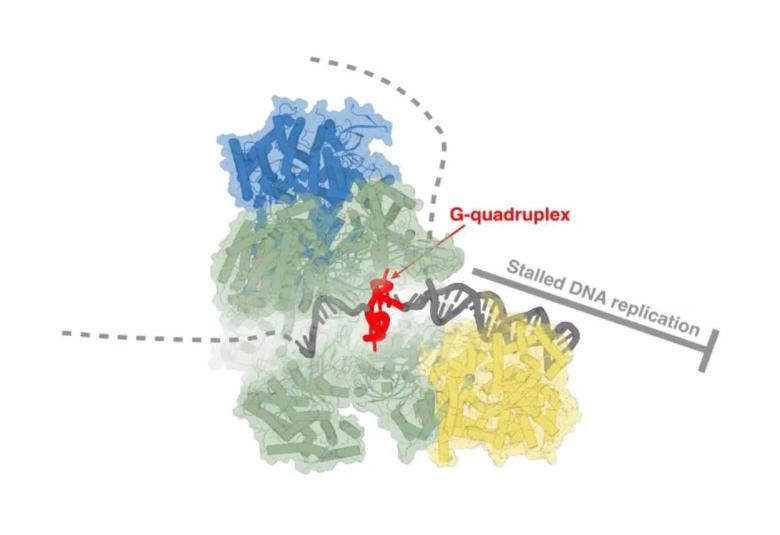
Cryo-Electron Microscopy reveals hidden mechanics of DNA Replication, sheds new light on cancer target
Every day, billions of cells in your body divide, helping to replace old and injured cells with new ones. And each time this happens, your entire genetic library your genome, which totals more than 3 billion base pairs of DNA has to be copied, precisely, from the parent cell to the new daughter cell.



 Vinay Kumar Singh.
Vinay Kumar Singh.