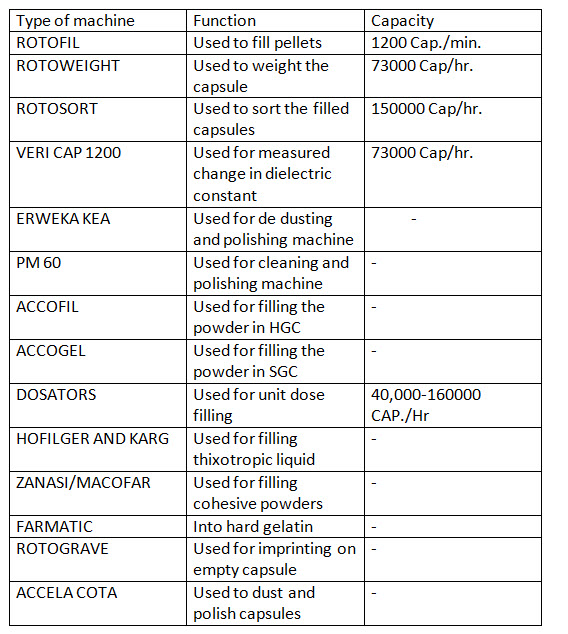
- SOFT GELATIN CAPSULE DEFINITION
- BASE ADSORPTION
- Composition of SGC
- STEPS OF MANUFACTURING OF SGC
- EVALUTION OF CAPSULE
- CAPSULE DEFECT
SOFT GELATIN CAPSULE
DEFINITION :- Soft gelatin capsule are one piece hermetically sealed soft gelatin shells containing liquid, suspension or semisolid.
Physic chemical properties of gelatin in SGC :-
1. BLOOM STRENGTH :- It is measure of cohesive strength of the cross-linking that occur between gelatin molecules, and it gives an indicator of the firmness of the gel. It is determined by measuring the weight in grams required to move a standard plunger that is 0.5 inches in diameter, a fixed distance of 4 mm deep into 6.66% W/V gel held at 10 C for 17 hours. Bloom strength in the range of 150-250 gm is considered suitable for capsule.
2. VISCOSITY :- It is determined using 6.66 % W/V of gelatin solution in water at 60 C using a capillary pipette. The viscosity range must be in 25-45 millipoise.
3. Iron content :- NMT 15 PPM
4. Formalin treatment :- result in decrease in solubility of gelatin film, because of cross linking of gelatin by aldehyde.
BASE ADSORPTION :-
• Formulation of suspension for SGC involves BA consideration.
BA = weight of base/ weight of solid.
• BA is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid.
minim per gram (M/G) =(BA+S)×V/W
• M/g factor is used mostly in vitamin formulation.
• BA is used to determine minim per gram factor of solid.
• M/G is volume in minim that is occupied by one gram of solid pulse weight of liquid base required to produce a capsutable mixture.
• Lower the BA of solid higher the density of mixture and thus smaller the capsule size.
• Best size and shape for convenient oral use in humans is 9 minims round,16 minim oval and 20 minim oblong.
Composition of SGC :-
• Gelatin :- Ideal substance for capsulation.
• Plasticizer :- Glycerin USP, Sorbitol USP, and their combination.
The ratio of dry plasticizer to dry gelatin measures the hardness of the capsule shell.

• Preservative :- Methylparaben : propylparaben (4:1), sorbic acid (0.2%)
• Colorants :- FD&C water-soluble dyes, certified lakes, pigments
• Opacifier :- Titanium dioxide
• Flavoring agent :- ethyl vanillin, essential oils.
• Fumaric acid :- To aid solubility and to reduce aldehyde tanning of gelatin.
STEPS OF MANUFACTURING OF SGC :-

EVALUTION OF CAPSULE
1. Weight variation :- 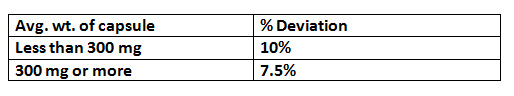
2. Content uniformity :- Test pass if all 30 capsule are within 75-123% range & NLT 27 of 30 capsule within 35-115 % range.
3. DISOLUTION TEST :- APPARTUST :- ROTATING BASKET TYPE
4. DISINTEGRATION TEST :-

• CAPSULE DEFECT :-
1. Soft sopt :- Due to slow drying during the formulation of shells, it occurs at the site in the shell, at which capsule lie next to the tray or against another capsule.
2. Dark sopt :- May occur in shell due to migration of water-soluble iron-sensitive ingredients from fill materials into shells.
3. Foreign capsule :- During sorting of the capsules, the unfilled capsules or different size capsules are called foreign capsule.
4. Bloating :- It is a defect where in the capsule seems to have widened/darkened and indentation of capsule with change in color hue. If capsule are stored at high humidity then the capsule size enlarges (bloated).









