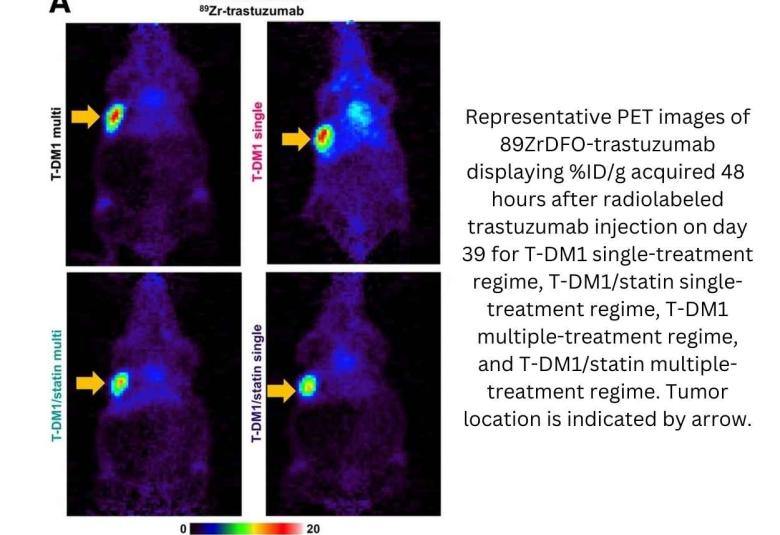
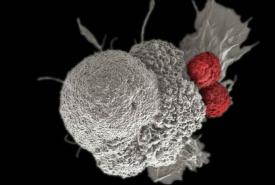
PET Imaging Validates use of Common Cholesterol Drug to Enhance HER2-Targeted Cancer Therapy
A novel therapeutic approach that combines human epidermal growth receptor factor 2 (HER2)-targeted therapies with the cholesterol-lowering drug lovastatin can reduce the number of cancer treatments required to prevent tumor growth. Monitored by immuno-PET scans, this combination therapy has the potential to personalize treatment for cancer patients and spare them from harmful side effects. This research was published in the October issue of The Journal of Nuclear Medicine.