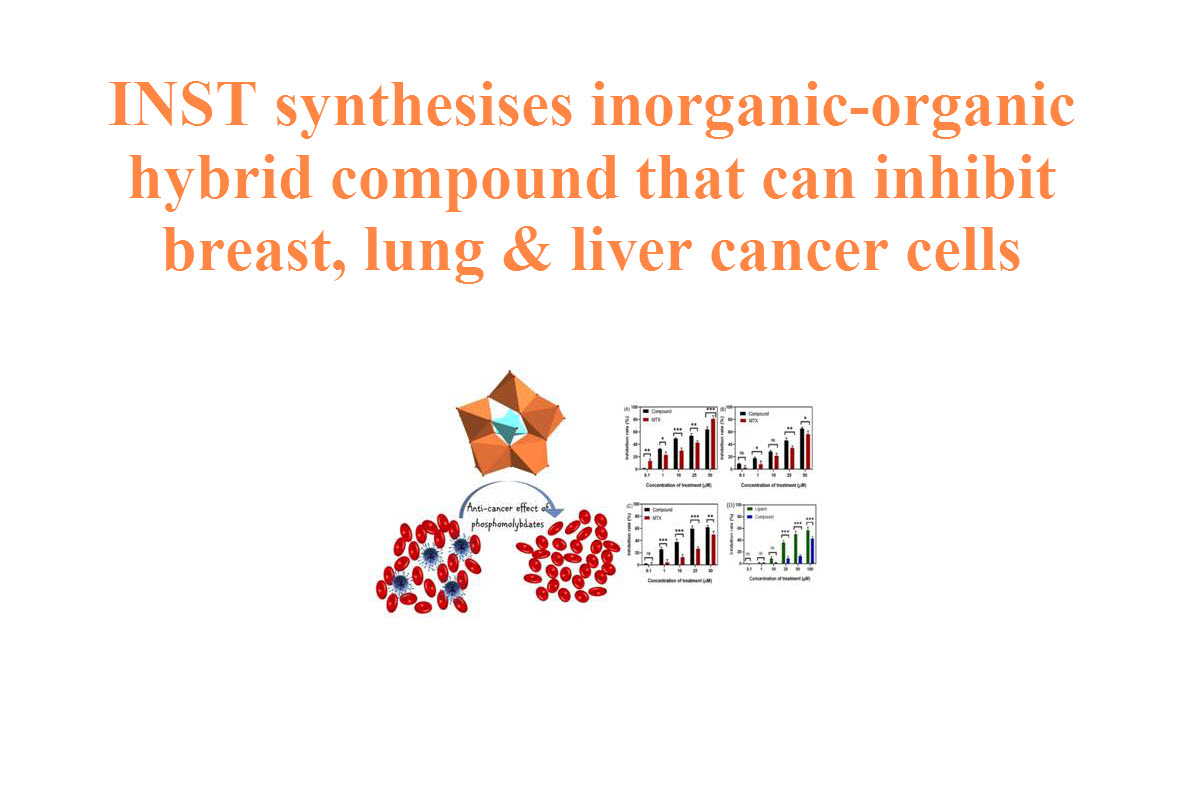
FDA Approves First-Line Immunotherapy for Patients with MSI-H/dMMR Metastatic Colorectal Cancer
U.S. Food and Drug Administration approved Keytruda (pembrolizumab) for intravenous injection for the first-line treatment of patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer. This marks the first immunotherapy approved for this patient population as a first-line treatment and which is administered to patients without also giving chemotherapy.