Researchers discover new hiding place for antibiotic resistance
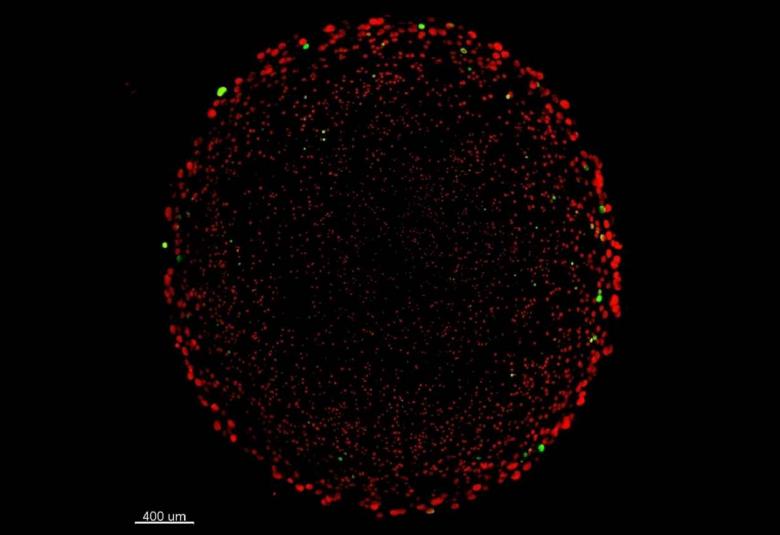
Antibiotic resistance is a race between us humans, who strive to find new antibiotics that can treat infectious diseases – and bacteria, which are becoming increasingly resistant. For now, bacteria are way ahead, which is why it is important for us to learn more about antibiotic resistance. A Danish research group has discovered a new piece of the puzzle that helps us better understand the 'enemy'.