A jump through time – new technique rewinds the age of skin cells by 30 years
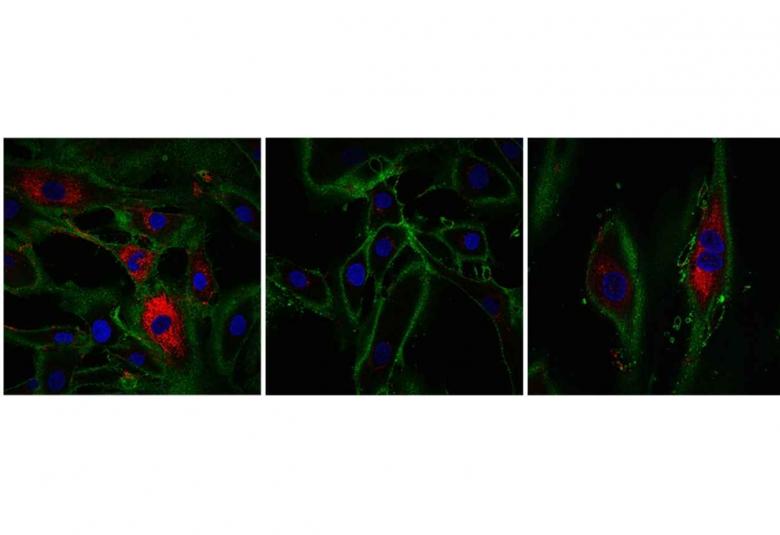
Research from the Babraham Institute has developed a method to ‘time jump’ human skin cells by 30 years, turning back the ageing clock for cells without losing their specialised function. Work by researchers in the Institute’s Epigenetics research programme has been able to partly restore the function of older cells, as well as rejuvenating the molecular measures of biological age. The research is published today in the journal eLife and whilst at an early stage of exploration, it could revolutionise regenerative medicine.