Ahmedabad based Troikaa Pharmaceuticals Ltd. has received patent for its novel non-aqueous Topical Solution of Diclofenac by the U.S. Patent Office which is marketed under the brand name Dynapar QPS.
Get the latest news from world and India’s leading pharmaceutical companies Pharma Industry, pharmaceutical marketing, generic drugs, and Complete news for Pharmacy and Life Sciences professionals.
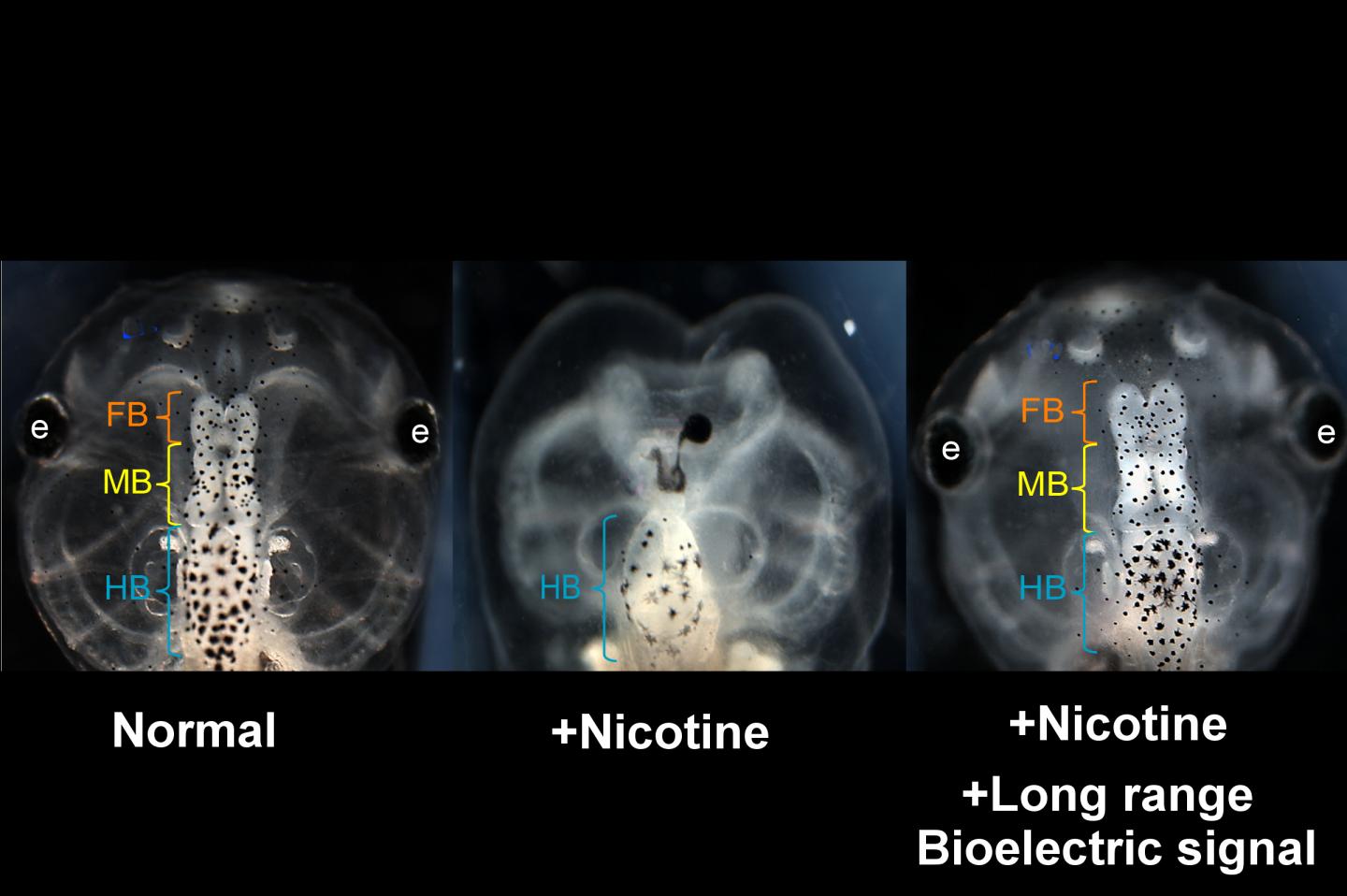
Caption : Nicotine induced defects in the frog embryo brain (center) can be rescued by transplanting an HCN2 expressing patch on the embryo far from the brain. Treated embryos are observed to have normal brain morphology and function (right). View of normal embryo head is shown at left. Similar results are seen when nicotine-exposed embryos are treated with ionoceutical drugs. (FB = forebrain; MB = midbrain; HB = hindbrain)
Researchers led by biologists at Tufts University have discovered that the brains of developing frog embryos damaged by nicotine exposure can be repaired by treatment with certain drugs called "ionoceuticals" that drive the recovery of bioelectric patterns in the embryo, followed by repair of normal anatomy, gene expression and brain function in the growing tadpole. The research, published today in Frontiers in Neuroscience, introduces intervention strategies based on restoring the bioelectric "blueprint" for embryonic development, which the researchers suggest could provide a roadmap for the exploration of therapeutic drugs to help repair birth defects.

Two novel biomarkers have been found to correlate with improved outcomes with immunotherapy in metastatic breast cancer and may help to identify the patients most likely to benefit from this treatment, according to exploratory studies reported at the ESMO Breast Cancer Virtual Meeting 2020. The biomarkers are an increase in the number of programmed death ligand-1 (PDL1/CD274) genes measured by copy number alteration (CNA) and the PD-L1 combined positive score (CPS), which assesses PD-L1 expression on both tumour and immune cells.
A research team led by investigators from Brigham and Women's Hospital has evaluated real-world evidence related to outcomes for COVID-19 patients who were treated with hydroxychloroquine or chloroquine analogues (with or without a macrolide). Investigators found no evidence that either drug regimen reduced the death rate among patients. Patients treated with hydroxychloroquine or chloroquine regimens were far more likely to experience abnormal, rapid heart rhythms (known as ventricular arrhythmias) than their counterparts who had not received the drugs. The team's findings are published in The Lancet.

The Union Minister of Health & Family Welfare Dr. Harsh Vardhan has been elected as Chair of the Executive Board of World Health Organization for the year 2020-21. This took place today during the 147th session of the Executive Board, in a meeting that was virtually held.Hehas replacedDr Hiroki Nakatani of Japan.

Cipla Limited announced that it has received final approval for its Abbreviated New Drug Application (“ANDA”) for Dihydroergotamine Mesylate Nasal Spray 4mg/mL from the United States Food and Drug Administration (U.S. FDA) with a Competitive Generic Therapy (“CGT”) designation. Cipla is the “first approved applicant” for such CGT and, is therefore, eligible for 180 days of CGT exclusivity which will begin to run from the commercial marketing of Cipla’s product. This 180-day CGT exclusivity will not block the commercialization of the existing approvals of Dihydroergotamine Mesylate Nasal Spray, 4 mg/mL.

Scientists have identified specific compounds from the Brazilian peppertree -- a weedy, invasive shrub in Florida -- that reduce the virulence of antibiotic-resistant staph bacteria. Scientific Reports published the research, demonstrating that triterpenoid acids in the red berries of the plant "disarm" dangerous staph bacteria by blocking its ability to produce toxins.
Indian researchers and authorities have prioritized to reduce dependence on other countries by developing and validating indigenous diagnostic assays for COVID-19 testing. A total of 11 RT-PCR-based indigenous assays were validated and recommended for the testing of COVID-19. This has been revealed by the Indian Council of Medical Research (ICMR).