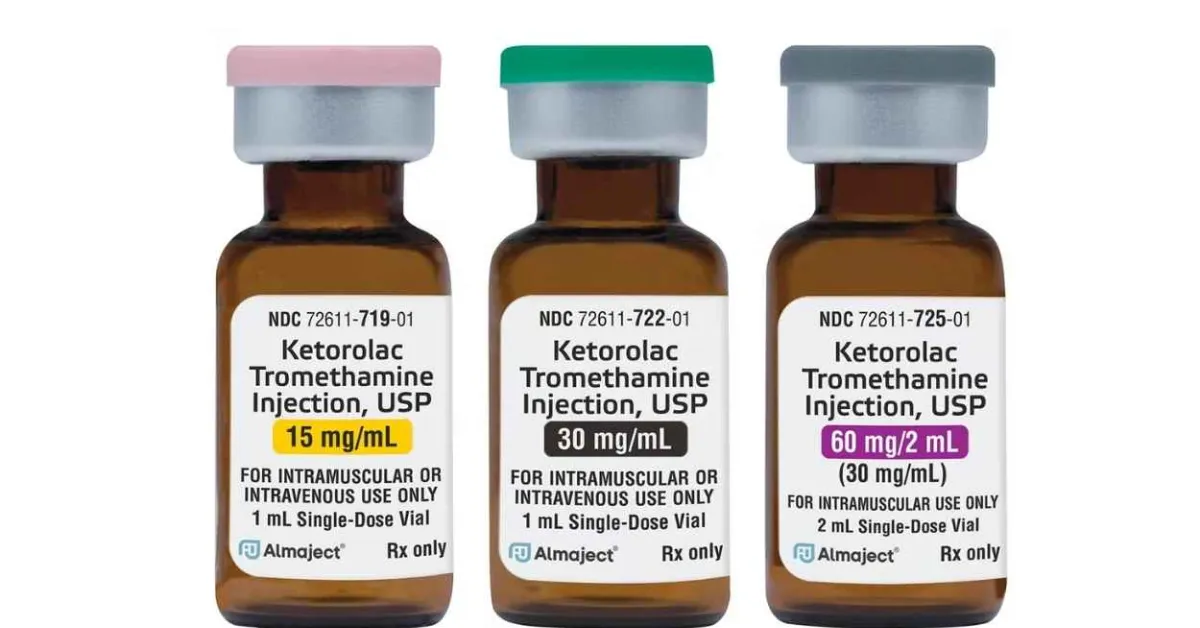
Alembic Pharmaceuticals Limited announced that it has received final approval from the US Food & Drug Administration (USFDA) for its Abbreviated New Drug Application (ANDA) for Ketorolac Tromethamine Injection USP, 15 mg/mL, 30 mg/mL, and 60 mg/2 mL (30 mg/mL) Single Dose Vials.
This is the second injectable product approval from our General Sterile Facility (F-3) which was inspected in August, 2022.
The approved ANDA is therapeutically equivalent to the reference listed drug product (RLD), Toradol Injection, 15 mg/mL, 30 mg/mL, and 60 mg/2 mL, of Roche Palo Alto, LLC (Roche). Ketorolac Tromethamine is indicated for the short-term (≤5 days) management of moderately severe acute pain in adult patients. Refer to our label for full indication.
Ketorolac Tromethamine Injection USP has an estimated market size of 59 million USD for twelve months ending June 2022 according to IQVIA.
Alembic has a cumulative total of 175 ANDA approvals (151 final approvals and 24 tentative approvals) from USFDA.