About Authors:
1Shambhawi, *Sai Saraswathi V
1*Pharmaceutical Chemistry Division,
School of Advanced Sciences,
VIT-University, Vellore-632014,
Tamilnadu, India.
1shambhawi06@yahoo.co.in, * v.saisaraswathi@vit.ac.in
ABSTRACT :
Standardization of polyherbal formulation is important to validate the quality of drugs and to ensure that the consumers are getting medication which guarantees purity, safety, potency and efficacy. The present paper reports standardization of traditional ayurvedic liquid polyherbal antidiabetic formulation (Sucrogen) and diabetes survey for retrieving the information on medication along with the lifestyle of diabetic population. Sucrogen was standardized based on ayurvedic pharmacopeia physico-chemical properties, preliminary phytochemical tests, organoleptic characters, stability studies, microbial studies, TLC, HPLC, heavy metal estimation by AAS and flame photometry to fix the quality standard of this drug. Invitro anti-diabetics activity of the drug was determined using alpha amylase Inhibitory method.These studies resulted in a set of diagnostic characters essential for its standardization. The phytochemical constituents found to be present in raw materials used for the preparation of Sucrogen possibly helps in achieving the desirable therapeutic efficacy of the ayurvedic formulation.
OBJECTIVE
To do a general survey on diabetes and to standardize the polyherbal formulation consumed by the population of Jharkhand for the determination of purity and quality of drug
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1418
INTRODUCTION
Diabetes is a chronic disease where the glucose levels in body increases due to either insufficient insulin production or utilization. There are different types of diabetes namely type 1 diabetes, type 2 diabetes, gestational diabetes, diabetes insipidus, canine diabetes feline diabetes etc. Here we are dealing with type 2 diabetes where the body produces plenty of insulin but the cells are unable to utilize it. According to the year 2010 in India 50.8 million people have acquired the disease and according to International Diabetes Federation (IDF) it is estimated that by year 2030, 80.4 million people will acquire this disease. As per World Health Organization (WHO) 50% population consume herbal drug for diabetes. Oral antidiabetic drugs and insulin therapy are available for its treatment, but a trend of shifting to herbal drugs have been seen among people due to its low cost, effectiveness and less side effects. So in this modern medicine era it is important to standardize traditional drugs to justify its acceptability. The polyherbal formulation i.e. Sucrogen was available from the market. It is manufactured by Joysree Kutir Silpam, Deoghar, Jharkhand and consumed by Jharkhand population.
SURVEY
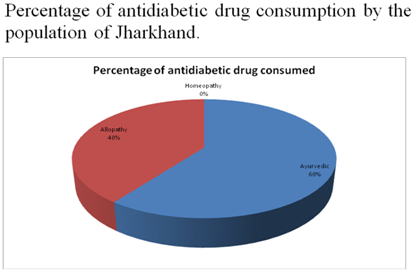
A general survey on diabetes in Jharkhand was done which gave us the idea of the medication and lifestyle followed by people of Jharkhand. It included online as well as field survey from hospitals and healthcares. The online link diabetessurvey.wordpress.com was made and was shared among our friends and relatives through mails and social network sites. Based on the result obtained we got to know the percentage of population having diabetes and whether they are undergoing ayurvedic, allopathy or homeopathy treatment and simultaneously ensured them the efficacy and purity of oral herbal drug.
STANDARDIZATION
To assess the quality of the formulation various standardization parameters studied were organoleptic, physicochemical properties, determination of pH, fluorescence analysis, preliminary phytochemical tests, density, viscosity, qualitative analysis by HPLC, TLC, microbial studies and estimation of metals by flame photometry, AAS. The organoleptic tests to determine the color, odor, taste and texture of the drug. Fluorescence studies will be carried to determine the characteristics under UV lamp.
MATERIALS AND METHODS
The formulation Sucrogen contains 13 ingredients which include:
|
Sanskrit/Hindi name |
Biological name |
Family |
Each 5ml contains |
|
Methi |
Trigonella foecum graceum |
Fabaceae |
200 mg |
|
Jamun beej |
Syzgium cumini |
Myrtaceae |
200 mg |
|
Satavari |
Asparagus racemosus |
Asparagaceae |
100 mg |
|
Aswagandha |
Withania somnifer |
Solanaceae |
100 mg |
|
Neem |
Azadirachta indica |
Meliaceae |
100 mg |
|
Guduchi |
Tinospora cordifolia |
Menispermaceae |
100 mg |
|
Gokshura |
Tribulus terrestris |
Zygophyllaceae |
50 mg |
|
Harida |
Curcuma longa |
Zingiberaceae |
50 mg |
|
Nayantara |
Catharanthus roseus |
Apocynaceae |
50 mg |
|
Piashal |
Terminalia tomentosa |
Combretaceae |
50 mg |
|
Triphala(Haritaki,Vibhitaki, |
Terminalia chebula, Terminalia bellirica, Phyllanthus emblica |
Combretaceae, Combretaceae, Phyllanthaceae |
50 mg |
|
Trikatu(Pippali,Maricha, |
Piper longum, Piper nigrum, Zingiber officinale |
Piperaceae, Piperaceae, Zingiberaceae |
50 mg |
|
Kalamegha |
Andrographis paniculata |
Acanthaceae |
50 mg |
All tests were performed following standard protocols.
Organoleptic Tests
The color, odor, taste and texture were reported taking 5 ml of sample.
pH
The pH test was performed by the pH paper and by pH meter method. Took the sample in a beaker and added a drop on the pH paper. The color change was compared with the standard and reported. For pH meter method, standard solution of pH 4 was taken. Then immersed the electrode in the beaker containing sample and noted the observed pH.
Density
The density of the formulation was found by the specific gravity bottle method. A clean dry specific gravity bottle was taken and empty weight was taken using (w1) Filled the bottle with distilled water and took the weight (w2). Then filled the bottle with the sample and took the weight (w3). The density was calculated using the formula
Density = w3- w1÷ w2- w1
Viscosity
Viscometer was used to determine the viscosity. A stock solution of 1% was made and from that 0.1, 0.2 and 0.3% concentration of a solution was made. The viscosities of these solutions were found concordant readings by the formula
ρ = ts ÷ t
Where, t is the time taken by water and
ts is the time taken by sample
Solubility
The solubility in various polar and non polar solvents was observed. The solvents included were water, ethanol, methanol, petroleum ether, hexane, benzene and chloroform.
Stability studies
The drug was subjected to stability testing by keeping at room temperature, accelerated temperature (107?C), cool and cold temperature.
Preliminary Phytochemical tests
The preliminary phytochemical qualitative tests were done following standard protocols.
Fluorescence analysis
Fluorescence characters of the sample were determined with different chemical reagents under fluorescence, short range UV, long range UV and daylight. The sample was taken on a watch glass and treated with various reagents for the presence of fluorescence characters.
Metal analysis
Estimation of calcium, and potassium by flame photometry
Made a stock solution of 200 ppm of each metal to be analyzed and diluted to solutions of 100, 80, 60, 40 and 20 ppm. First calibrated with the standard solutions taking dilution factor as 1 and then estimated the sample. From the graph obtained reported the concentration of calcium, and potassium.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Estimation of zinc, magnesium and iron by atomic absorption spectrometer
Nitric acid digestion
The sample was subjected to nitric acid digestion a day before. The sample and ml of concentrated nitric acid in a boiling tube was subjected to a temperature of 90?C for 45 minutes and then added ml of concentrated nitric acid. Kept the boiling tube on sand bath at a temperature of 150?C for 8 hours till the sample becomes clear.
Preparation of standard solutions
Zn (100 ppm)
Made the stock solution by dissolving 0.44 g of ZnSO4 and 1ml 5M CH3COOH in 100 ml of distilled water. Dilutions of 20, 40, 60 and 80 were made.
Mg (10 ppm)
0.5 g and concentrated HCl to dissolve and made up the volume to 100 ml. Dilutions of 2, 4, 6, 8 was made.
Fe (10 ppm)
0.7022 g of FAS and 2.5 ml of 1M H2 SO4 and made upto 100 ml. Dilutions of 2, 4, 6, 8 was made.
The calibration with standard solutions and estimation of the metal concentration in sample was done using Varian AA 240 AAS
HPLC (Waters 1525 binary HPLC pump)
Mobile phase: A solvent system of methanol- water in the ratio 50:50 was used.
Flow rate: 1ml/min
Injection volume: 20µl
Detection at: 230nm
Column used: C18
TLC
Made silica plates and added the sample 2cm from the edge of plate. Saturated the walls of the beaker with the solvent system. Kept the plates in the mobile phase of methanol and water (50:50). Then the plates were developed in iodine chamber and observed in UV chamber.
Microbial studies
The formulation was subjected to microbial test to ensure that it is free from microorganisms. The petri plates, swabs, nutrient agar media was autoclaved at 121?C for 30 minutes. The media poured in the petri-plate and allowed to solidify. Inoculated the media with the formulation. Kept for incubation at 37?C for 24 hours keeping one plate as control. Observed the plates for growth of microorganisms.
Amylase inhibitory test
500µl of sample and 500µl of phosphate buffer- alpha amylase incubated at 25?C for 10 minutes. Added 500µl of 1% starch solution and incubate for 20 minutes at 25?C. 1 ml of DNS reagent was added and kept in boiling water bath for 5 minutes. Cooled at room temperature, added 10 ml of distilled water and measured the absorbance at 540 nm.
% of amylase inhibitory = (Absorbance of solvent with enzyme- absorbance of solvent) - (absorbance of sample-absorbance of blank) ÷ (Absorbance of solvent with enzyme- absorbance of solvent x 100
RESULTS
Organoleptic properties
Color: dark brown
Odor: bitter
Taste: bitter
Texture: fine
pH
The pH was found to be 5 to 6 by pH paper and 5.8 by pH meter.
Density
Weight of empty bottle (w1) = 25.1305g
Weight of water + bottle (w2) = 52.1811g
Weight of sample + bottle (w3) = 52.8514g
Density= w3- w1÷ w2- w1
= 52.8514-25.1305 ÷ 52.1811-25.1305
= 1.02 g/ml
Viscosity
|
Concentration (%) |
Trial 1 (sec) |
Trial 2 (sec) |
Trial 3 (sec) |
Concordant reading (sec) |
|
0.1 |
1.40 |
1.41 |
1.41 |
1.41 |
|
0.2 |
1.44 |
1.43 |
1.44 |
1.44 |
|
0.3 |
1.47 |
1.47 |
1.48 |
1.47 |
|
Water |
1.59 |
1.58 |
1.59 |
1.59 |
Viscosity= Time taken by sample ÷ Time taken by water
|
Concentration (%) |
Viscosity (Pa.s) |
|
0.1 |
0.8867 |
|
0.2 |
0.9056 |
|
0.3 |
0.9245 |
Solubility
|
Water |
Soluble |
|
Ethanol |
Soluble |
|
Methanol |
Soluble |
|
Petroleum ether |
Not Soluble |
|
Hexane |
Not Soluble |
|
Benzene |
Not Soluble |
|
Chloroform |
Not Soluble |
Stability studies
The formulation was found stable at room, accelerated and cold temperature.
Preliminary Phytochemical tests
|
Alkaloids |
+ |
|
Glycosides |
- |
|
Saponins |
+ |
|
Carbohydrates |
- |
|
Sterols |
- |
|
triterpenoids |
+ |
|
Flavanoids |
+ |
|
Proteins and amino acids |
+ |
|
Tannins |
+ |
Fluorescence analysis
|
Sample+reagent |
Day light |
Fluorescence |
Short UV |
Long UV |
|
Conc. HCl |
Brown |
Brown |
Yellow |
Green |
|
Conc. HNO3 |
Brown |
Yellowish brown |
Purple |
Yellowish green |
|
CCl4 |
Brown |
Brownish yellow |
Greenish yellow |
Yellowish green |
|
CH3 COOH |
Brown |
Brown |
Purple |
Green |
|
NH3 |
Brown |
Brownish yellow |
Yellow |
Greenish yellow |
|
FeCl3 |
Brownish yellow |
Reddish yellow |
Bluish yellow |
Yellow |
Metal analysis
|
Metals |
Concentration |
|
Potassium |
15 ppm |
|
Calcium |
4 ppm |
|
Zinc |
1.4648 ppm |
|
Magnesium |
0.4415 ppm |
|
Iron |
0.0207 ppm |
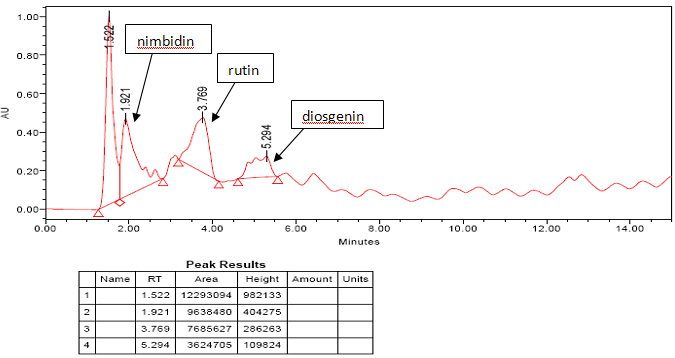
HPLC
TLC
The spots were obtained at Rf value 0.47 and 0.63 which corresponds to the standard value of 0.5 for rutin (5) and 0.66 for diosgenin(6).
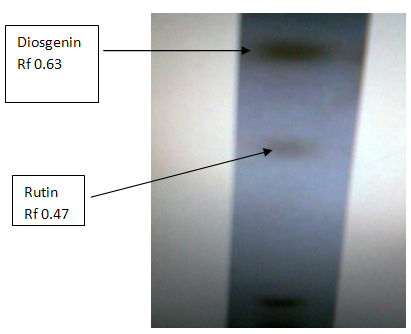
Fig: TLC plate of showing rutin and diosgenin
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Microbial studies
No growth was seen for the plate inoculated with drug.
Petriplate inoculated with drug showed no growth of microrganisms
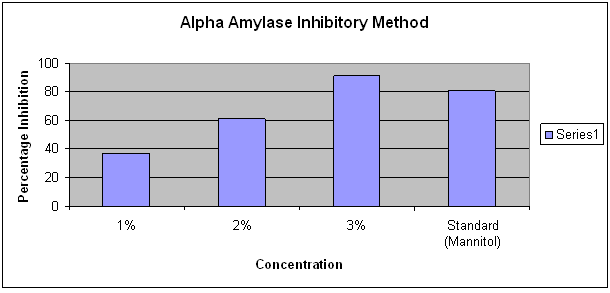
Amylase inhibitory test
The sample showed inhibition of amylase enzyme with increase in concentration.
|
Sample |
Absorbance |
|
Blank |
0.1447 |
|
Solvent (water) |
0.0390 |
|
Solvent with enzyme |
0.0761 |
|
Test solution 1(1%) |
0.1682 |
|
Test solution 2(2%) |
0.1591 |
|
Test solution 3(3%) |
0.1480 |
|
Standard |
0.1518 |
|
Concentration |
% of amylase inhibition |
|
1% |
36.65 |
|
2% |
61.18 |
|
3% |
91.10 |
|
Standard (Mannitol) |
80.8 |
DISCUSSION
From the survey done in Jharkhand, it was found that majority of population are consuming ayurvedic drug Sucrogen which is effective and does not possess any side effects. The polyherbal formulation Sucrogen was subjected to various analytical techniques. The organoleptic parameters revealed that dark brown color, bitter taste and odor and fine texture of the formulation. The pH was found to be between 5 and 6. The solubility in water, ethanol and methanol shows that it is soluble in polar solvents.The phytochemical tests are in accordance with the ayurvedic pharmacopeia standards.Presence of tannins and alkaloids were the supporting evidence for antidiabetic property of Sucrogen. Fluorescence is a significant phenomenon exhibited by various chemical constituents in plants. The substances can be derivatized by reagents if they are not themselves fluorescent hence some crude drugs are often assessed qualitatively in this way and it is an important parameter of pharmacognostical evaluation.Metal estimation showed the presence of potassium, calcium, zinc, magnesium and trace amount of iron. HPLC result showed a peak at 1.921, 3.769 and 5.294 minutes which corresponds to the peak of nimbidin, rutin, and diosgenin respectively which is in accordance with the standard values(7,8,9). Nimbidin is an active constituent of neem. Diosgenin, a saponin is found in Methi. Rutin is a flavanoid found in jamun beej. All these three components possess antidiabetic property. In TLC Rf values are obtained at 0.47 and 0.63 respectively for rutin and diosgenin. The plate inoculated with the drug showed no microbial growth hence the drug is free from contamination. The amylase enzyme inhibition was observed with increase concentration of drug hence antidiabetic property of the formulation was confirmed invitro.
CONCLUSION
Ayurvedic formulation Sucrogen has been standardized as per the WHO guidelines and ayurvedic pharmacopeia. Based on the survey as well as standardization done it has been found that Sucrogen has good antidiabetic property and can be used for evaluating the quality and purity of formulations for polyherbal drug.
REFERENCE
1. N.V Sathesh Madhav, Kumud Upadhayaya, Asha Bisht, (2011), Phytochemical screening and standardization of polyherbal formulation for dyslipidemia, International journal on pharmacy and Pharmaceutical Sciences, Vol 3 Supp 3.
2. Neeli Rose Ekka, Kamta Prasad Namdeo and Pradeep Kumar Samal,(2008) standardization strategies of herbal drugs-an overview Research J. Pharma and Tech 1(4):page 310-312.
3. Kartik Ch patra, K Jayaram Kumar, P Suresh, (2009) standardization of polyherbal siddha formulation amukkara choornam, International journal of traditional knowledge Vol 8(3), pp.449-452.
4. Soni Hardik K, Ribadiya Nikunj, Bhatt Surendra B, Sheth Navin R, (2010) Evaluation of herbal formulation (capsule) containing ashwagandha as a single herb with their nutritional value determination, International Journal of Applied Biology and Pharmaceutical Technology, vol 1: issue 3.
5. Brett J. West and Shixin Deng, (2010), Thin layer chromatography methods for rapid identity testing of Morinda citrifolia L.(Noni) Friut and leaf, advance journal of food science and technology 2(5): 298-302.
6. Hisham Y.Al-Matubsi, Nagham A.Nasrat, Ghaleb A.Oriquat, Mahmoud Abu-Samak, Khtan A.Al-Mzain and Maher Salim, (2011), The hypocholesterolemic and antioxidative effect of dietary diosgenin and chromium chloride supplementation on high –cholesterol fed Japanese quails, Pakistan journal of biological sciences, 14(7) 425-432.
7. Amitava ghosh, Piyali chakrabarti, Partha roy, Somnath bhadury, Tanusree nag and Simli sarkar (2009), Bioremediation of heavy metals from neem(Azadirachta indica) leaf extract by chelation with dithizone. Asian journal of pharmaceutical and clinical research, volume 2 issue 1.
8. Willy shah, Nilan rane, M. B. kekare and Vikas vaidya, (2010), Estimation of two bioactive compounds from azadiracta indica A. juss. Leaves using HPLC, international Journal of Pharma and biosciences. Vol 1(2).
9. Mohamed Z. Gad, Maha M. El-Sawalhi, Manal F. Ismail and Nibal D. El-Tanbouly, (2006), Biochemical study of antidiabetic action of the Egyptian plants fenugreek and balanites Molecular and Cellular Biochemistry 281: 173–183.
10. K R Gopala Simha, V.Laxminarayana, SVLN Prasad, Shahjahan Khanum, (2008) standardization of yogaraja gugullu- an ayurvedic polyherbal formulation, International journal of traditional knowledge, vol7(3), pp.389-396.
11. P.Stanley Mainzen prince, N.Kamalakkannan,(2006), Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes, Journal of biochemical and molecular toxicology, vol 20,96-102.
12. Vijayaraghavalu Sai Saraswathi, Dhakshanamurthy Thirumalai, Pothula kushal Yadav and Muniswamy saranya,(2011), pharmacognostic and preliminary phytochemical study of Lagerstroemia speciosa leaves, international journal of research in ayurveda and pharmacy, vol2(3) 893-898.
13. Nasreen.S(2011), Evaluation of preformulation and formulation parameters of an antistress herbal capsule, international journal of Pharma and biosciences,vol2.
14. Arun kumar beknal, Prakash g korwar, M.A.Halkal, Upendra kulkari, Basawaraj S.Patil, Srinivas R.Soodam(2010), Phytochemical investigation and antioxidant activity study of drynaria quercifolia linn rhizome, International journal of current pharmaceutical research, vol2 36-39.
15. Manisha modak, Priyanjali dixit, Jayant londhe, Saroj ghakadbi, and Thomas paul A.Devasagayam,(2006), Indian herbs and herbal drugs used for the treatment of diabetes.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









