{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Rakshita A.S, Shashank Nayak N, Shwetha S Kamath K
Bapuji Pharmacy College,
Davanagere, Karnataka, India
ABSTRACT
Paracetamol belongs to the class of antipyretic and analgesic. Various brands of some dosage forms are available in the market with a common claim that they are all bioequivalent. The main objective of the present experiment was to evaluate post compression parameters of various brands of paracetamol tablets containing 650 mg of drug and to determine whether all the formulations used were equivalent or significantly different. Paracetamol of 650 mg standard tablets from different manufacturers were selected in the studies. The post compression parameters like weight variation, friability, hardness, weight variation, disintegration, dissolution profiles were found to be varying but within the prescribed limit.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2645
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 7, Issue 02 Received On: 11/01/2019; Accepted On: 16/01/2019; Published On: 01/02/2019 How to cite this article: Nayak, S., Rakshita, A.S., Shwetha, S. and Kamath, K. 2019. Study of Post Compression Parameters of Various Marketed Paracetamol Tablets in India. PharmaTutor. 7, 2 (Feb. 2019), 35-42. DOI:https://doi.org/10.29161/PT.v7.i2.2019.35 |
INTRODUCTION
Paracetamol is a non steroidal anti-inflammatory drug .It is prominently used as antipyretic and anodyne(analgesic), In treatment of fever, pain ,headache and such other discomfort. Its chemically familiar as acetaminophen i.e., (4- hydroxy acetanilide). It is also given in combination with many cold medication, also in cancer pains and to reduce the pain after surgery. It is predominantly safe in normal doses, higher doses causes hepatic disorders.(Nayak AK 2010)
Evaluations of different parameters like, hardness, friability, weight variation ,disintegration time dissolution profile were performed. Therapeutic effectiveness and bioavailability of tablet depends on these parameters(Kar A et al.,2015)(D.R.Jadge et al., 2014) .Depending on this facts the present study was conducted to compare the quality standards of different commercially available paracetamol 650 mg tablets.
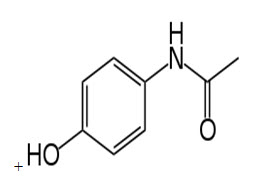
Fig No: 1- Chemical Structure of paracetamol4
POST COMPRESSION EVALUATION PARAMETERS
WEIGHT VARIATION
This is an important in process quality control test ,which has to be checked frequently (every half hour ). Corrections are made during the punching of tablet if necessary. Any variation in the weight of tablet (for any reason )leads to either under medication or overdose .This is particularly true when the drugs are potent or low dose drugs .All tablet machines have provision to receive a known quantity (volume which is correlated to weight )of granules. Improper flow of granules from the hopper into the die is responsible for weight variation . The range of variation is 10 % for tablets weighing less than 80 mg, 7.5% for tablets weighing in the range of 80 to 250 mg, 5.0 % for tablets weighing above 250 mg. For paracetamol the deviation allowed is 5 %.
FRIABILITY
Friability is the loss of weight of tablet in the container/ package ,due to removal of the particles from the surface .This in process quality control test is performed to ensure the ability of tablets to withstand the shocks during processing (coating and strip.- packaging ) handling ,transportation and shipment(Rahman Z U et al.,2013)(Binega G et al.,2013). The friability of tablets is indicated by chipping, capping, breaking. The extent of friability is less than 0.8% .This limit is strictly adhered when the tablets are further processed for coating.
HARDNESS
Hardness is a force required to break a tablet across the diameter. The hardness of the tablet is an indication of its strength. This is a valuable test which might influence tablet disintegration and dissolution rate.
DISINTEGRATION
Disintegration is defined as that state in which any residue of tablet except fragments of insoluble coating ,remaining on the screen of the test apparatus consisting of a soft mass having no palpably firm unmoistened core .Disintegration process involves the breaking of tablet into small particles .The quicker the disintegration the faster could be the action .disintegration roughly indicates the possible pattern of dissolution of active substance . hence the experimental conditions closely mimic the situation that a tablet encounters in GI tract , in terms of temperature ,ph and mechanics.
DISSOLUTION
Dissolution is defined as “the amount of drug substance that goes into solution per unit time under standardized conditions of liquid/solid interface, temperature and solvent composition” . Dissolution is conducted to know the minimum time taken by the drug to dissolve itself in the systemic circulation and starts its action in the body. There are 2 methods of to know the dissolution rate,
1.In vitro studies
2. In vivo studies .
In the pharmaceutical industry, drug dissolution testing is routinely used to provide analytical in vitro drug release erudition for both quality control scheme, i.e., to compute batch-to-batch consistency of solid oral dosage forms such as tablets and drug reinforcement, i.e., to anticipate in vivo drug release profiles. It serves as are presentative factor for bioavailability and bioequivalence.
Hence comparative dissolution studies of various commercially marketed paracetamol products. paracetamol tablets of 650mg were selected .
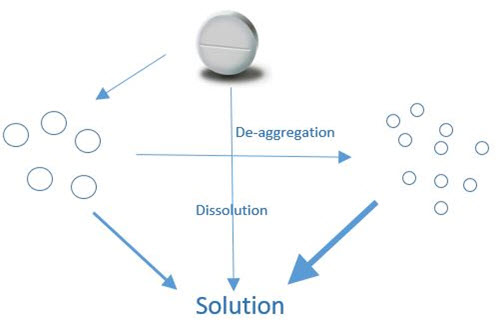
Fig No : 2 – Dissolution studies
Applications of dissolution
1) Dissolution test is used as a quality control tool to scrutinize habitually the uniformity and reproducibility of production batches. In case , a single point determination i.e., a certain percentage of the drug dissolved (released) in a given time interval is usually a sufficient control parameter.(Siew A 2016)
2) Dissolution test is used as a exploration tool for augment the parameters and ingredients in a new formulation. Commonly multi point determination is employed .
3) However in vitro and in vivo co-relations are surveyed ,there the dissolution studies are used as means to surrogate the recurring studies of absorption .Normally multipoint determination is selected.
Factors influencing the dissolution
The important experimental factors that influence the dissolution of drugs are listed below :
Temperature(body temperature ) - 370 C ( 36.50 to 37.50 C)
Agitation (hydrodynamics) - 25 to 150 rpm (normally 50 Revolutions per minute)
Dissolution media (environment of GIT) - water ,0.1 N hydrochloroic acid, Simulated gastric fluids , Simulated intestinal fluids
These are fixed in order to mimic the dissolution in the biological system. When the test is used as quality control tool ,a rapid method is desirable .Accordingly experimental conditions are fixed.
MATERIALS AND METHODS
The various marketed paracetamol tablet containing 650 mg ( Medamol 650 mg ,Paracip 650 mg,P-650 mg, Crocin 650 mg , paracetamol (janushad)650 mg and Dolo 650 mg.
WEIGHT VARAITION
Tablets of each brand were weighed individually using a analytical balance .20 tablets were weighed individually,average weight is calculated from the total weight .The percentage difference in the weight variation should be within the permissible limits.
FRIABILITY
Roche friability(D.R.Jadge et al.,2014) is used to measure the friability of the tablets .it rotate at rate of 25 rpm .10 tablets are weighed collectively and placed in the chamber of the friability. In the friabilator the tablets are exposed to rolling ,resulting from free fall of tablets within the chamber of the friabilator .After 100 rotation (4 minutes )the tablets are taken out from the friabilator and intact tablets are again weighed collectively . Percentage friability is determined by using this following formula .
Friability = (W1-W2/W1) *100
Where as W1= weight of the tablets before test
W2= weight of the tablet after test
HARDNESS
The hardness of tablet is tested using the Monsanto hardness tester . The tester is placed across the diameter in between the spindle and the anvil . the knob is adjusted to hold the tablet in position .The reading of the pointer is adjusted to zero . the pressure is increased slowly to break the tablet . “Hardness factor” - the average of the several determination is determined and reported
DISINTEGRATION
It was performed using Electro Lab Disintegration apparatus, 12 tablets were taken in disintegration test apparatus at 37± 2 C containing simulated gastric fluid (0.1N HCl). Noted down the time taken for tablets to disintegration .
DISSOLUTION
Various marketed paracetamol tablets containing 650 mg of drug was purchased from local pharmacy in Davanagere. All the other ingredients are of analytical grade.
Preparation of buffers
Preparation of phosphate buffer solution, pH 7.8
Five hundred ml of 0.2M potassium dihydrogen is taken in 2000ml volumetric flask, to which 445.0ml of 0.2 N sodium hydroxide solution is added and the volumetric flask to which 445.0 ml of 0.2 N sodium hydroxide solution is added and the volume is made up to the mark with distilled water.
Potassium dihydrogen phosphate (0.2 M) solution ;
Potassium dihydrogen phosphate (13.609 gram)is added to 500 ml volumetric flask containing distilled water and the volume is made up to the mark with distilled water.
Sodium hydroxide ( 0.2N) solution :
Four gram of sodium hydroxide is taken in 500 ml volumetric flask containing distilled water and volume is made up to the mark with distilled water.
PROCEDURE
The dissolution method is a kinetic method .i.e., periodical samples are withdrawn for the determination
1) Phosphate buffer solution pH 7.8(900ml ) is measured and transferred into the dissolution flask.
2) The temperature is maintained at 37±0.50 C.
3) Various marketed paracetamol tablet containing 650 mg drug is placed at the bottom of the jar.
4) The paddle is rotated at 50 rpm .
5) Five ml of sample is withdrawn at 0 min and transferred into the test tube appropriately labeled. Immediately 5ml of phosphate buffer solution pH 7.8 is replaced into the dissolution flask .
6) Similarly samples are collected at 1,2,3,4,5,6,7,8,9,10 min intervals. A 5 ml of fresh dissolution medium is replaced in the flask whenever the sample is withdrawn .
7) All samples are filtered using wattman filterpaper .
8) The absorbance at 249 nm is measured in uv spectrophotometer using the phosphate buffer solution as a blank.
9) The absorbance are recorded in the table and further calculations are done
10) A graph is plotted by taking cumulative percent of drug dissolved on y-axis and time on x-axis .

Fig No:3 – Dissolution apparatus
RESULTS AND DISCUSSIONS
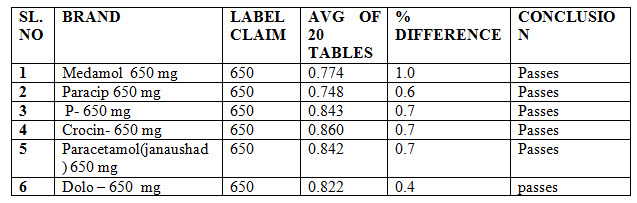
WEIGHT VARIATION : The weight variation of various brands of paracetamol are shown below in table no :1
Table No1- Weight variation studies of marketed paracetamol products
FRIABILITY : The friability of various brands of paracetamol are shown below in the table no 2
Table No 2- Friability data of marketed paracetamol products

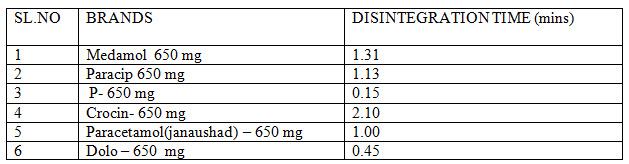
DISINTEGRATION: The disintegration of various brands of paracetamol is shown below in table no :3
Table No 3- Disintegration data of studies of marketed paracetamol products
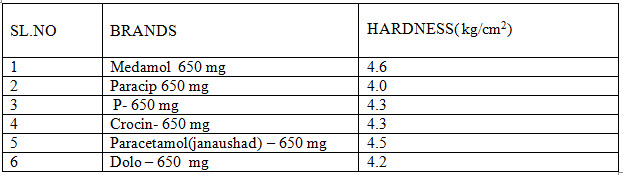
HARDNESS :
Hardness of various marketed brands of paracetamol are shown below in table no :4
Table No 4-Hardness studies of marketed paracetamol products
DISSOLUTION
The dissolution profile of marketed tablets like Medomol 650, Paracip 650, P-650, Crocin 650 and Paracetamol 650 mg (Janaushad) are listed in the following table.
Table No :5 -Drug release profile of Medamol 650 mg tablet

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table No : 6-Drug release profile of Paracip 650 mg tablet

Table No :7- Drug release profile of P-650 mg tablet

Table No : 8 - Drug release profile of Crocin 650 mg
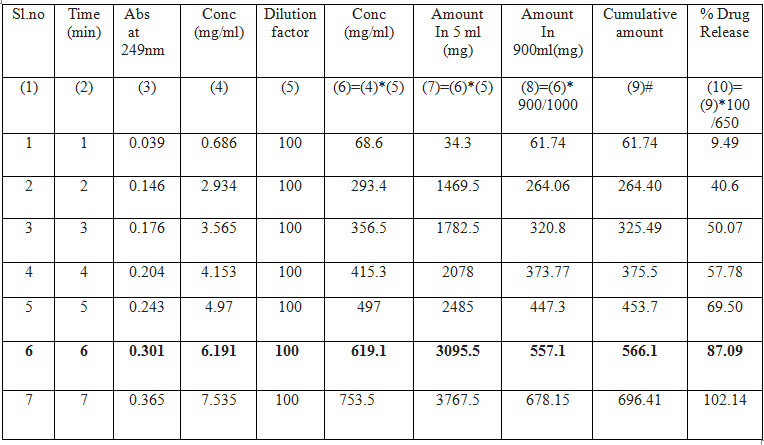
Table No : 9 - Drug release profile of Paracetamol 650 mg (janaushad)

Table No : 10 – Drug release profile of Dolo 650 mg
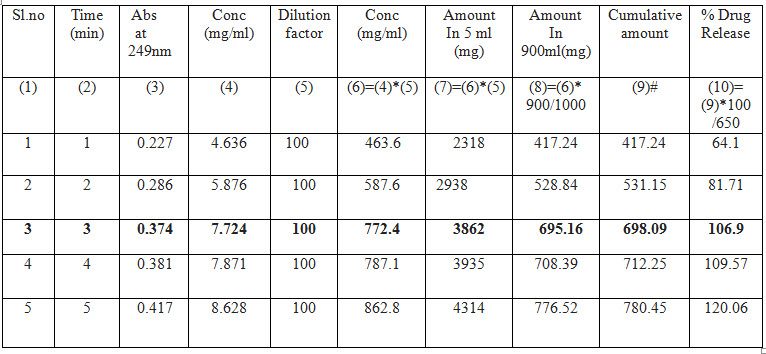
Table No : 11 - Comparative drug release profiles of Paracetamol tablet

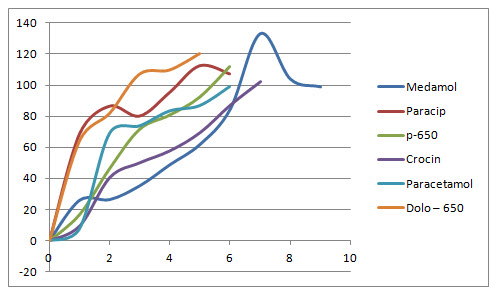
Fig No :4 -Dissolution profile of six different brands of paracetamol tablets
CONCLUSION
As a result of this study we have concluded that all the six brands of Paracetamol tablets meet the criteria laid in the official monographs and though they differ slightly in terms of various parameters like weight variation, hardness, friability, shows its complete release at in the range of 4 to 7 mins .All marketed paracetamol tablets of 650 mg were all under specified IP limits.
REFERENCES
1. Binega G and Wondimsigegn D et al.,(2013); “Analysis and comparison of paracetamol tablets dispensed in legal dispensaries and non pharmaceutical shops in Gondar town ,north west ,Ethiopia”; Int J of Phar and Ind Res ;3(2); 144-151
2. D.R Jadge and A.N Despande et al., (2014); “Comparative invitro evaluation of different brands of paracetamol tablets marketed in Maharashtra state”; Int J of Pharma Sci and Health Care; 4(4) ; 40-7
3. https://www.drugbank.ca/drugs/DB00316 ( Cited on 17/12/18 at 2.45 PM)
4. Kar A and Amin MN et al., (2015) ; “Quality analysis of different marketed brands of paracetamol available in Bangladesh” ; Int Cur pharm J; 4(9); 432-5
5. Mathur N and Kumar R et al., (2015); “ Evaluation of quality control parameters on various brands of paracetamol tablet formulation” ;World J of Pharm and Pharma Sci ;4 (7); 976-984
6. Nayak AK (2010); “Comparative in vitro dissolution assessment of some commercially available paracetamol tablets”; Int J of Pharma Sci Review and Res ; 2(1); 29-30.
7. Rahman ZU and Khan I et al.,2013; “Post-Market In-Vitro Comparative Evaluation of Quality Control Parameters of Paracetamol Compressed Tablets Manufactured in Local Industrial Zones of Kpk Pakistan”; The Pharma innovation J ; 2(3); 11-5
8. Siew A (2016) ; “dissolution testing” ;Pharma Tech ;40(11); 56-64
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









