{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
M. Jambulingam*, S. Ananda Thangathurai, D. Kamalakannan, S. Punitha, Rincy T.R, S. Santhi, G. Surya, M. Vasanthi., S. Josephine Subarla.
Department of Pharmaceutical Analysis, Swamy Vivekanandha College of Pharmacy,
Elayampalayam-637205, TN, India
*jambulingam48@gmail.com
ABSTRACT:
A simple spectrometric method has been developed for the estimation of the ceftriaxone sodium in powder for injection dosage form by derivatization with p-dimethyl amino benzaldehyde. The measurement of absorbance and derivatized ceftriaxone sodium at 490.4nm. The both methods obeys Beer’s and Lambert’s law in the range of 5-25µg/ml with the correlation co-efficient of r²0.998. The colour reaction was highly stable and didn’t show any changes in absorbance up to 48hrs. The % RSD associated with all the validation parameter was less than 2, showing the accuracy of the method developed. The compliance of acceptance criteria of Q2 (R1), (R2) international conference on harmonization (2005 guidelines).
INTRODUCTION:
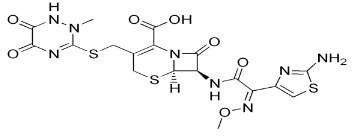
Ceftriaxone Sodium is chemically (6R,7R)-7-[[(2Z)-(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-3-[[(1,2,5,6-tetrahydro-2-methyl-5,6-ioxo-1,2,4–triazin-3-yl)thio]-methyl]-5-thia-1 azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid[1].
Ceftriaxone is a third generation semi synthetic cephalosporin antibiotic. Cephalosporin’s are derivatives of 7-amino cephalosporic acid and are closely related to penicillin’s in structure. Ceftriaxone sodium is a long acting, broad spectrum cephalosporin antibiotic for parenteral use. Inhibition of cell wall synthesis. It widely active against gram negative and gram positive microorganism, both penicillinases and cephalosphrinases are highly stable to beta lactamases.
Several analytical methods have been reported for the analysis of Ceftriaxone sodium based on spectrophotometric derivatives[2], FIA[4], flourimetric[5], thinlayerchromatographic[6], ion selective electrodes[7], ion exchange chromatographic[8], high performance ion pair liquid chromatography[9], polar graphic techniques[10].
For spectrometric analysis the determination is carried out using suitable reagents such as 10% sulphuricacid, ethanol, 5% Paradimethylaminobenzaldehyde, toluene, water and methanol. The derivation either increases the molar absorptivity or produces bathochromatic shifts in the absorbance. Therefore 5% Para diethyl amino benzaldehyde is reported for the first time as a derivatizing reagent for spectrophotometric determination of Ceftriaxone sodium in pharmaceutical preparation.
CHEMICAL STRUCTURE OF CEFTRIAXONE SODIUM:
MATERIALS AND METHODS:
|
S.NO |
INGREDIENTS |
SUPPLIER |
|
1 2 3 4 5 6 7 |
Ceftriaxone sodium p-dimethyl aminoBenzaldehyde Sulphuric acid Toluene Methanol Ethanol Water |
Hospirapvt.Ltd. Chennai Nice chemicals pvt .Ltd. cochin Loba chemicals pvt.Ltd. Mumbai Ranbaxy fine chemicals pvt.Ltd. Mumbai Loba chemicals pvt.Ltd. Mumbai Loba chemicals pvt.Ltd.Mumbai Double distilled water |
APPARATUS AND INSTRUMENTS:
Double beam UV-visible spectrophotometer Perkin Elmer lamda model lamda 25.
System controller: UV probe 2.31
Mode: Spectrum
Scan speed: Medium
Wavelength range:400-800nm
Weighing balance: SHIMADZU electronic balance ELB series
Volumetric flask of 10,100&1000ml(borosil)
Pipettes of 1, 2, 5&10ml (borosil)
Preparationof standard stock solution:
A stock solution of ceftriaxone sodium were prepared by dissolving accurately weighed 100mg of pure drug in distilled water in 100ml volumetric flask to get concentration of 1000µg/ml. The above solution is further diluted with distilled water to get a concentration of 100µg/ml and were kept as stock solution.
Preparation of 5% p-dimethyl amino benzaldehyde (PDAB):
Weigh 5gm of p-dimethyl amino benzaldehyde in a 100ml of methanol in a 100ml volumetric flask to obtain the desired solution.
PREPARATION OF DERIVATIZED COMPOUND:
The derivatized solution was prepared in a 10ml volumetric flask and add 2ml10%sulphuricacid,2ml PDAB,2drops of toluene and ethanol to get a set of solution having concentration in the ranging from 2-50µg/ml for ceftriaxone sodium solution the working solution were scanned at 490.6nm respectively.
METHOD DEVELOPMENT:
DETERMINATION OF λmax:
The derivatized solution of ceftriaxone sodium were scanned in the wavelength region of 400-800nm.The λmax was found to be 490.6nm and the spectrum was shown in the figure No: 1
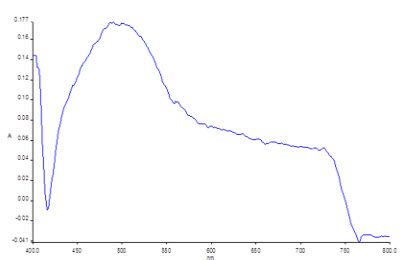
FIGURE NO:1 λmax OF CEFTRIAXONE SODIUM
PREPARATION OF CALIBRATION CURVE:
Appropriate aliquots were pipette from each standard stock solution into a series of 10ml volumetric flask. The volume was made up to the mark with 2ml 10%sulphuricacid,2ml PDAB,2drops of toluene and ethanol to get a set of solution having concentration range of 2-50µg/ml for ceftriaxone sodium solution. The working solution was scanned at 490.6nm respectively. The absorbance was recorded and plotted against the concentration to obtain their calibration curve.
PREPARATION OF SAMPLE SOLUTON:
Sterile ceftriaxonesodium powdered equivalent to 1gm/ml of ceftriaxone sodium was dissolved in a 10ml double distilled water and sophisticated for 10min.Further 1ml of the ceftriaxone sodium wastransferred into 100ml of volumetric flask and the volume was make with water .The sample stock solution further derivatized with 10%sulphuricacid, 2drops of toluene,5%PDAB and the volume was made with ethanol to get a finalconcentration in the linearity range.
METHOD VALIDATION:
As per ICH guidelines Q2 (R1), the method validation parameters were studied: Accuracy, Linearity and Precision, Limit of detection and Limit of quantization[3].
LINEARITY:
Ceftriaxone sodium absorbance of standard solution (5- 25µg/ml) was measured at visible spectrophotometer at 490.6nm.Absorbance for the drug was plotted against their respective concentration to get correlation co-efficient greater. The correlation coefficient was shown in the figure 2.
PRECISION:
The precision (intraday precision)were evaluated by measuring three independent samples of ceftriaxone sodium in pure form at three different concentration levels in pharmaceutical formulations. The standard deviations and percentage standard deviation were in the range of 20 µg/ml and 40µg/ml,0.103 and 0.105 and 0.3% and 0.3% respectively. In the same manner, the assay for precision (interday precision) at each concentration levels repeated for 3 days. The values of standard deviation and percentage standard deviation 0.102 and 0.104 and 0.3% and 0.3% respectively. The values of standard deviation and relative standard deviation can be considered to be very satisfactory and thus the proposed methods are very effective for the determination of ceftriaxone sodium in pure form and sterile formulations was shown in the table no: 2&3.
ACCURACY
The accuracy of the method were evaluated with the percent recovery, standard deviation and percentage standard deviation by using standard addition method. Three levels of standard drug were individually add with the 1g equivalent of powder for injection dosage form ceftriaxone sodium were analysed in four replicates. The results obtained for methods through the standard addition method shown in table(no: 4) that the standard deviations.
LIMIT OF DETECTION AND QUANTIFICATION:
Limit of detection (LOD) and the limit of quantification (LOQ) were calculated using the standard deviation of intercept (σ) and slope (s) of the calibration curve.
LOD=3.3×σ/S
LOQ=10×σ/S
Where,
σ= the standard deviation of the response and
s= slope of the calibration curve.
LINEARITY:
TABLE: 1 REGRESSION CHARACTERIZES
|
PARAMETER |
CEFTRIAXONE SODIUM |
|
Linearity range (µg/ml) Linearity equation LOD(μg/ml) LOQ(μg/ml) Correlation coefficient(r) Molar absorptivity Sandell’s sensitivity |
5-25 Y=mx 3.3(SD/S)=0.0217µg/ml 10(SD/S)=0.0640µg/ml 0.998 5.7 ×103
5 |
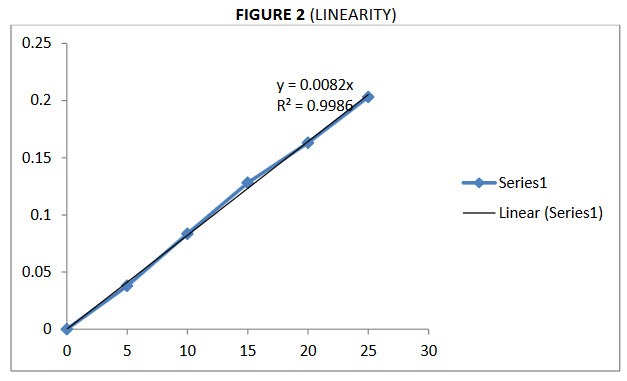
FIGURE: 2 (LINEARITY)
PRECISION:
TABLE: 2 INTRADAY PRECISION DATA FOR CEFTRIAXONE SODIUM
|
S.NO |
CONCENTRATION (µg/ml) (n=3) |
CEFTRIAXONE SODIUM ABSORBANCE |
||
|
|
20µg/ml |
40µg/ml |
20µg/ml |
40µg/ml |
|
1 2 3 |
3 3 3 |
3 3 3 |
0.143 0.150 0.148 |
0.204 0.209 0.198 |
|
MEAN STANDARD DEVIATION %RSD |
0.147 0.103 0.3 |
0.204 0.105 0.3 |
||
TABLE: 3 INTERDAY PRECISION DATA FOR CEFTRIAXONE SODIUM
|
S.NO |
CONCENTRATION(µg/ml)(n=3) |
CEFTRIAXONE SODIUM ABSORBANCE |
||
|
|
20µg/ml |
40µg/ml |
40µg/ml |
|
|
1 2 3 |
3 3 3 |
3 3 3 |
0.205 0.206 0.198 |
|
|
MEAN STANDARD DEVIATION %RSD |
0.146 0.102 0.3 |
0.203 0.104 0.3 |
||
TABLE: 4 ACCURACY
|
S.NO |
LEVEL |
AMOUNT PRESENT (µg) |
AMOUNT ADDED (µg) |
AMOUNT RECOVERED |
%MEAN RECOVERY |
|
1 2 3 |
80% 100% 120% |
8 10 12 |
8 10 12 |
15.85 19.70 23.92 |
99.06 98.5 99.6 |
TABLE: 5 ANALYSIS OF MARKETED FORMULATION
|
S.N0 |
BRAND NAME |
LABEL CLAIM |
AMOUNT PRESENT |
% RECOVERY |
|
1 2 3 |
NOSOCEF CEFEX CEFTRON |
1000mg 1000mg 1000mg |
9963mg 9976mg 9989mg |
99.63 99.76 99.89 |
RESULT AND DISCUSSION:
It reacts with nitrogenous substance in presence of mineral acid to forms adduct compounds. It is used for color development in colorimetry.The acid is used for the maintained of acidic condition.
TABLE: 6 STUDENT T TEST AND F TEST
|
S.NO |
BRAND NAME |
PROPOSED METHOD |
REFERENCE METHOD |
STUDENT t TEST |
F VALUE |
|
1 2 3 |
NOSOCEF CEFEX CEFTRON |
99.6 99.76 99.89 |
99.9 98.6 99.4 |
0.0001 0.0020 0.0024 |
37.64 46.34 47.76 |
# Significance P < 0.005
Derivatized ceftriaxone sodium showed absorbance maximum at 490.4nm (fig.no.1) at this wavelength did not show any significant absorbance of the excipients. Therefore it was carried out for analysis.
The linearity of absorbance versus concentration was demonstrated by linear least square regression analysis. The linear equation was y=0.998. Beer’s law obeyed over the concentration range of 5-25µg/ml, molar absorpitivity and sandell’s sensitivity are very high which indicate the sensitive of the method LOD and LOQ values of drug and all calibration were less than 2% (%RSD) which indicates the accuracy of the method.
Accuracy of the method was carried out by applying the standard dilution techniques in the mean percentage recovery between actual concentration and taken concentration for ceftriaxone sodium was calculated.
To check precision assay were carried out within a day and in three consecutive days by three different concentrations of the analyte %RSD values were 0.2 and 0.4 respectively.
Ceftriaxone sodium sterile formulations were analyzed by proposed method and by reported method which involved analyst with 20,40,µg/ml at measured absorbance.
Result are compared with F-test and student t-test it show 95% confidence level and for four degree of freedom.(Table.6) which shows the accuracy of the proposed method.
CONCLUSION:
In the present proposed method, we have developed simple ,precise and accurated quantitative estimation of ceftriaxone sodium in bulk and sterile preparation by UV-spectrophotometric method. Hence this method can be used for routine analysis of ceftriaxone sodium in bulk and sterile preparation formulations.
ACKNOWLEDGEMENT:
The authors are very much thankful to the management and faculty of Swamy Vivekanandha College of Pharmacy, Elayampalayam for providing support to carry out this work.
REFERENCES:
1. A.H Beckette, J.B Stenlake: Practical Pharmaceutical Chemistry. CBS publishers and distributors, Newdelhi, 4th edition, part2.48&378
2. S.D Seth: Text book of pharmacology. A division of reed Elsevier pvt Ltd, Newdelhi, 3rd edition, 41
3. ICH-guidelinesQ2A: Validation of analytical procedures. Definition and determinology Geneva, Switzerland, 1995, 68-76
4. Chatwal: Textbook of pharmaceutical analysis. Himalaya Publishing House, Delhi, 5th edition, 39-45
5. Gallen W. Wing, McGrew Hill: Textbook of instrumental analysis. Himalaya Publishing House, Delhi, 2nd edition, 48&378.
6. K.D Tripathi: Text book of Essential of medical pharmacology. 7th edition, 2013,681,712,726, 727
7. K. Ilango, P.Valentina: Text book of medicinal chemistry. 1st edition, 2007.Vol 2, 133 &137.
8. Patel K.R., Patel V.D., Patel K.P. and Patel V.G; Development and validation of spectrophotometric method for determination of ceftriaxone sodium in pharmaceutical dosage forms; Derpharma. chemica; 2010; 2(5); 255-260
9. Al-Momani I.F; Spectrophotometric determination of selected cephalosporin’s in drug formulations using flow injection analysis; J. Pharmaceutical and Biomedical Analysis; 2001; 5(6); 751-757
10. Pal N., Rao A.S. and Hedi M.A; HPLC method development and validation for the assay of ceftriaxone sodium injection; International. J. Pharma.Sci; 2012; 2(4); 84-90
REFERENCE ID: PHARMATUTOR-ART-2357
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 3, Issue 9 Received On: 18/04/2015; Accepted On: 28/04/2015; Published On: 01/09/2015How to cite this article: M Jambulingam, SA Thangathurai, D Kamalakannan, S Punitha, TR Rincy, S Santhi, G Surya, M Vasanthi, SJ Subarla; A Simple spectrophotometric estimation of ceftriaxone sodium in bulk and sterile formulation; PharmaTutor; 2015; 3(9); 48-52 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









