 About Authors:
About Authors:
Kambham Venkateswarlu1*, G.Sujatha2
1Graduate Student
2Department of Pharmaceutical Chemistry
Sri Lakshmi Narasimha College of Pharmacy, Palluru,
Chittoor District, Andhra Pradesh-517132, India.
*k.v.reddy9441701016@gmail.com
I. INTRODUCTION
1.1. WHAT IS PROSTATE CANCER?
Prostate cancer is the most common type of cancer in men and is the second leading cause of cancer-related deaths in men worldwide. It is a disease of the prostate gland in which malignant cells form in the tissues of the prostate and multiply out of control. These cells often metastasize to other parts of the body, like the rectum, the bladder and especially, the bones and the lymph nodes. It generally arises near the surface of the gland so that it can be diagnosed easily by digital rectal examination (DRE). Depending on the extent of metastasis, the prostate cancer tissue has been graded using the Gleason System into a score of 2 to 10 or into four stages I-IV (or, A-D), indicating the likelihood of spreading of the disease to other parts of the body.
Although several types of cells are found in the prostate, over 99% of the cancers occur in the gland cells only (cells which make the prostate fluid which is added to the semen). Such type of cancer is termed as adenocarcinoma. The region of prostate gland where the adenocarcinoma is most common is the peripheral zone. Other types of cancer can also start in the prostate gland, including sarcomas (like leiomyosarcoma and rhabdomyosarcoma), small cell carcinomas, and transitional cell carcinomas, although they are extremely rare. Generally, the cancer grows and spreads very slowly. Early prostate cancer most often causes no symptoms. Sometimes, however there are signs and symptoms very close to those of benign prostatic hyperplasia (BPH).
Reference Id: PHARMATUTOR-ART-1605
1.2. ANATOMY AND FUNCTION OF THE PROSTATE GLAND
The prostate, a part of the male mammalian reproductive system, is a firm, partly glandular, partly fibromuscular body, surrounding the beginning of the male urethra. It lies at a low level in the lesser pelvis, behind the inferior border of the symphysis pubis and pubic arch and anterior to the rectal ampulla, through which it may be palpated. It is an exocrine gland about the size of a walnut and consists of three lobes: one centre lobe with two lobes on each side. The prostate is traversed by the urethra and ejaculatory ducts, and contains the prostatic utricle. The urethra usually passes between its anterior and middle thirds. The ejaculatory ducts pass antero-inferiorly through its posterior region to open into the prostatic urethra.
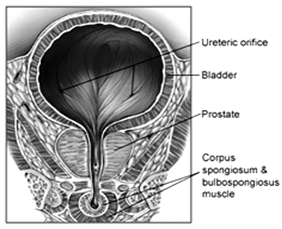
Figure 1. Location of the prostate in the male reproductive system
As part of the male reproductive system, the primary function of the prostate gland is to secrete a slightly alkaline fluid (pH 7.29) that constitutes 10-30% of the seminal fluid, a fluid that carries sperm. During male climax (orgasm), the muscular glands of the prostate help to propel the prostate fluid, in addition to sperm, that was produced in the testicles, into the urethra.
Valve-less venous communication between the prostatic and extra-dural venous plexuses normally occurs, probably an important factor in the metastasis of prostatic neoplasms to the vertebral bodies.
1.3. EPIDEMIOLOGY OF PROSTATE CANCER:
Prostate cancer is the commonest form of carcinoma afflicting men the world over. In the US, it is the most rampant of all the cases of cancer in men and is the second leading cause of death after lung cancer.
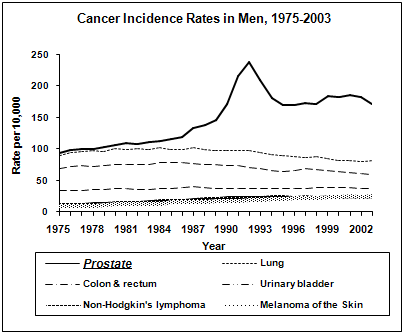
The North American Association of Central Cancer Registries (NAACCR)has estimated that 29% of the incident rates and 9% of the death rates in males from all types of cancer can be attributed to prostatic carcinoma alone. The disease is mainly related to older age as it is mostly diagnosed in men above 40. Also, Asians have the lowest incidence rates of the diseasewhereas the African-Americans are more likely to present with advanced disease than the other races, as per the results of the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute (NCI).
1.4. SIGNS AND SYMPTOMS OF PROSTATE CANCER
Prostate cancer is most cases it is accompanied by no symptoms at all. The symptoms however are not specific to the disease; they could be caused by an enlarged prostate or BPH and include frequent urination, or weak or interrupted urine flow, pain or burning during urination, blood in the urine or semen, frequent urge to urinate mainly in the night, difficulty in achieving erection or painful ejaculation. Once the cancer has spread to other parts of the body it may manifest itself in the form of bone pain, back pain, urinary and faecal incontinence,weight loss and leg weakness due to compression of the spinal cord by spreading of cancer in the spine.
1.5. PREDISPOSING FACTORS OF PROSTATE CANCER
The specific and exact cause of the disease is still unknown but can be divided into:
1.5.1. Endogenous Factors
a. Age – The risk of developing prostate cancer increases manifold with advancing age. Men more than 65 years of age are more predisposed to the disease than younger ones. The average age at the time of diagnosis is 70.
b. Race/Ethnicity – The disease has a high predisposition to racial factors. The African-Americans are at the highest risk whereas Asians are at the lowest risk with the Hispanics and the Whites in between.
c. Family history - Prostate cancer occurrence has also been demonstrated to have a familial aggregation, with a two to four-fold increased risk of the disease among men who have reported prostate cancer in a close relative like father or brother.
d. Hormones - Circulating androgens like dihydrotestosterone (DHT) and testosterone probably play an important role in the initiation and promotion of the disease and also in the proliferation of cancerous cells in the prostate cancer.
e. Genetic Factor – A man’s genetic background also contributes to the risk of developing the disease. About 44% of patients with prostate cancer have a genetic component. Specific genetic lesions resulting in prostate cancer are uncommon though some genes have been implicated in the progress of the disease like the BRCA1, BRCA2 and a high risk gene on chromosome 17p called ELAC2.
1.5.2. Exogenous Factors
a. Diet – Dietary amounts of certain foods, vitamins, and minerals can contribute to prostate cancer risk. Men with higher serum levels of the short-chain ω-6 fatty acid linoleic acid have higher rates of prostate cancer. Other dietary factors that may increase prostate cancer risk include low intake of vitamin E, omega-3 fatty acids, and selenium.
b. Environmental agents – Many agents like cadmium, infectious substances and environmental carcinogens have been implicated in the development of the disease. Japanese and Chinese men who live in the West, mainly the US, develop a high rate of the disease than their Asian counterparts.
c. Medications – There are also some links between prostate cancer and medications, medical procedures, and medical conditions. Daily use of anti-inflammatory medicines such as aspirin, ibuprofen, as also the hypolipidaemic drugs like the statins may decrease prostate cancer risk.
d. Lifestyle – It has been reported that the degree of sexual activity and/or fertility may govern the risk of developing the disease.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
1.6. ANDROGEN RECEPTOR – AN INTRODUCTION
The normal development and maintenance of the prostate gland is dependent on androgens acting through the Androgen Receptor (AR), which is a member of the steroid and nuclear receptor superfamily. It is most closely related to the progesterone receptor (PR).7 It is officially designated as NR3A and is a ligand-activated transcription factor which controls the biological responses of androgens.
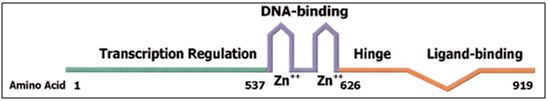
Figure 2. Structure of the AR
The receptor is a protein having 919 amino acid residues with a molecular mass of 110 kDa. The receptor is the only steroid receptor encoded by a gene on the X-chromosome (Xq11-12). The gene is known as the AR gene.
1.7. AR AND PROSTATIC DEVELOPMENT: ROLE IN GROWTH OF PROSTATE CANCER:
The prostate comprises many endocrine glands that secrete components of the seminal fluid into ducts that empty into the urethra. Secretary epithelial cells line the ducts and these in turn are surrounded by a layer of basal epithelial cells, a basement membrane and stromal cells.
During development, androgen-dependent growth of the prostate occurs. This requires AR expression in the stromal cells, which are believed to secrete factors causing proliferation of the epithelial cells in response to androgens. At maturity, androgen stimulated growth of the prostate ceases and androgens are required to maintain the prostate and promote its secretory function, which requires AR expression in the luminal epithelial cells. In cases of benign or malignant overgrowth of the prostate, androgen-dependent growth inappropriately resumes, and in the case of prostate cancer, this hyperproliferation usually occurs in the luminal epithelial cells of the ducts.
Also, mutations in the AR gene have been evidenced in various cell lines including the Lymph Node Carcinoma of Prostate (LNCaP) cell line. Improved techniques with potentially increased sensitivity for detection of point mutations have yielded results which suggest a higher frequency of base substitutions in the AR coding sequence of untreated advanced prostate cancers and in hormone-relapsed tumors from primary and metastatic sites. The LNCaP cell lines express AR with a T877A point mutation that causes proliferation in the presence of the antiandrogens hydroxyflutamide and cyproterone acetate. This mutation is also found in patients with antiandrogen withdrawal syndrome (AWS).
Two LBD mutations, W741L and W741C have been reported, that occurred upon androgen deprivation and bicalutamide treatment of LNCaP cells. These are thought to be mainly responsible for the AWS, where refractory prostate cancers regress after discontinuation of antiandrogen administration.
1.8. PROSTATE CANCER THERAPY
Different types of treatment are available for patients with prostate cancer. Some treatments are standard and some are being tested in clinical trials. Four types of standard treatment are used:
a. Watchful waitingalso known as Observation, Expectant Therapy or Deferred Therapy
b. Surgeryincluding Pelvic Lymphadenectomy, Radical Prostatectomy, Retropubic Prostatectomy, Perineal Prostatectomy, Transurethral Resection of the Prostate (TURP)
c. Radiation Therapyusing high-energy X-rays to kill cancer cells
d. Hormone Therapyis a cancer treatment that removes hormones or blocks their action and stops cancer cells from growing. This may include LHRH agonists like leuprolide, antiandrogens like cyproterone acetate (steroidal) or bicalutamide (non-steroidal), estrogens, androgen-synthesis blockers like ketoconazole etc.
e. Cryosurgeryor cryotherapy where prostate cancer cells are frozen and killed
f. Chemotherapywhich isa cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing
g. High-Intensity Focused Ultrasound (HIFU)which uses ultrasound waves to destroy affected cells
h. Biologic Therapyor biotherapy uses patient’s immune system to fight cancer
II. BACKGROUND
2.1. MECHANISM OF ACTION OF THE ANDROGEN RECEPTOR
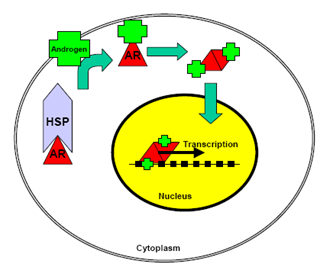
Figure 3. Mechanism of action of the AR
Unliganded AR is sequestered in the cytoplasm in a complex with members of the heat shock protein (HSP) family of chaperones, and high-molecular weight immunophilins. Indeed, in normal prostate tissue and most PCa cell lines, the AR is predominantly cytoplasmic under castrate conditions. The chaperone complex serves to induce a high-affinity conformation in the AR that is competent for ligand binding. Once bound to ligand, there is a change in the composition and conformation of the AR-chaperone complex, which exposes the bipartite AR nuclear localization signal, thus allowing translocation of AR to the nucleus. Agonist-bound AR is highly mobile in the nucleus and engages with androgen response elements (AREs) in the promoter and enhancer regions of genes critical for the growth and survival of normal and cancerous prostate cells. The prototype AR-regulated gene, PSA, encodes prostate-specific antigen PSA, a secreted serine protease, which is a major component of seminal fluid and an important biomarker for PCa development and progression. The mechanisms of AR-mediated transcription of the PSA gene have been studied extensively and have revealed that ARE-bound AR recruits a plethora of coregulatory proteins, which play important roles in the initiation of transcription, as well as the fine-tuning of overall transcriptional output.
2.2. STRUCTURE AND FUNCTION OF VARIOUS DOMAINS OF THE AR
The AR shares an overall modular organization similar to other steroid receptors, having an N-terminal domain (NTD) [1-557], harbouring AR transcriptional activation function (AF)-1, a central DNA binding domain (DBD) [558-625], a short hinge region [626-676], and a COOH terminal domain (CTD) [677-919], which contains both the AR ligand-binding domain (LBD) [677-870] and AF-2 Co-activator binding surface. The three-dimensional structure of peptides representing the LBD and AF-2 folds of the AR have been determined by X-ray crystallography, as has the three-dimensional structure of a peptide representing the AR DBD. The AR NTD, is unstructured in solution due to its high flexibility and intrinsic disorder in solution, though biophysical study has revealed that it exists in a molten globule conformation.
Post translational modifications of the AR, including phosphorylation, acetylation, ubiquitylation etc. add additional layers of regulation and are likely to influence the structure and function of these domains.
2.2.1. Structure and Function of the AR CTD
The role of the AR CTD is of particular importance for prostate cancer, because the AR CTD is the foremost target of current androgen ablation therapies. Antiandrogens like bicalutamide bind the LBD block the activity of AF-2 and cause AR to recruit Corepressor molecules such as nuclear receptor corepressor (NCoR) to the promoters of AR-regulated genes. Overall, these result in the prevention of AR activity, thus inhibiting the growth and survival of androgen-dependent PCa cells.
The three-dimensional structure of the AR LBD is very closely related to those of many other nuclear receptor LBDs which reveal highly similar globular domains made up of twelve α-helices, which organise as three antiparallel helical sheets. AR also has a similar structure though composed of eleven α-helices in place of the twelve, due to the absence of helix 2. A total of eighteen amino acid residues, situated within each of the eleven AR LBD helices, are responsible for direct interactions with the ligand, with helix 12 functioning as a flexible lid to stabilize the ligand within the LBD cavity.
Binding of ligand to the AR LBD causes a significant positional change in helix 12, as well as an overall conformational change in the AR CTD. This induces formation of the AF-2 coactivator binding surface. The AF-2 surface is a hydrophobic groove flanked by concentrated regions of positive and negative charge. However, the relative role of the AR AF-2 in coactivator recruitment remains unclear.
Additionally, another important role may exist for the AR AF-2. For instance, in addition to serving as a docking site for coactivators, AR AF-2 is able to mediate interaction with the AR NTD in an intramolecular fashion. From a functional standpoint, this N/C interaction is essential for various binding strategies. Moreover, AF-2 preferentially binds the NTD when the AR is mobile, but binds coregulators when the AR engages with DNA. These findings suggest that N/C interaction may block inappropriate coregulator interaction until AR is engaged with AREs in the promoter and enhancer regions of the target genes. After AR is bound to DNA, the AF-2 cleft may be more amenable to coregulator binding, which would enhance the transcriptional activity of the AR.
2.2.2. Structure and Function of the AR DBD and Hinge Domains
The AR DBD/Hinge region plays important roles in mediating AR nuclear localization, receptor dimerization, and DNA binding. Hormone receptor DBDs are highly conserved, consisting of two zinc fingers and a loosely structured carboxy-terminal extension (CTE). The first zinc finger contains the P-box, which is the recognition helix that binds the DNA major groove. The second zinc finger contains the D-box, which is the AR dimerization interface.
2.2.3. Structure and Function of the AR NTD
The AR NTD accounts for more than 60% of the AR protein and can be envisioned as regions of rigid secondary structure which exists as a molten globule conformation. It functions as a potent transcriptional activator independent of the CTD. This NTD activity generally referred to as AR AF-1, contrasts with those of the AR AF-2 which are quite weak in comparison. AR AF-1 is thought to be the major domain responsible for mediating AR transcriptional activity.
2.3. ANTIANDROGENS
Antiandrogens, by definition, antagonize the action of testosterone or 5α-DHT by competing for AR binding sites. It is but logical that since an essential step in the action of androgens in target cells is binding to the AR, so for neutralizing the androgens, antiandrogens are most useful. Since prostate cancer is very highly sensitive to androgens, the antiandrogen used should have a high specificity and affinity for the AR while not possessing any androgenic, estrogenic, progestational, glucocorticoid or any other hormonal or antihormonal activity. The mechanisms of antiandrogen action may be either directly by interaction with the AR or indirectly through some nonreceptor-mediated action or metabolism or nonspecific antimetabolite activity. The antiandrogens are very useful in the treatment of prostate cancer, benign prostatic hyperplasia (BPH), acne, virilization in women and male contraception. Currently, there are two main classes of antiandrogens20 that are clinically utilized:
i. Steroidal antiandrogenswhich have the typical perhydrocyclopentanophenanthrene skeleton. A few steroidal ligands have been used as antiandrogens, including cyproterone acetate, oxendolone, and spironolactone. However, the clinical application of steroidal antiandrogens has been limited greatly by poor oral bioavailability, a lack of tissue selectivity, poor pharmacokinetic properties and potential side effects like hepatotoxicity, androgenic effects and feminizing side effects like gynecomastia and loss of libido in men. Moreover, the rigid steroid backbone does not leave room for further structural modifications for future drug development.
ii. Non-steroidal antiandrogens: The current pharmacological treatment of choice for progressive androgen-dependent prostate cancer is the nonsteroidal antiandrogens, either as monotherapy or with adjuvant castration or luteinizing hormone-releasing hormone (LHRH) superagonists (like goserelin) to block the synthesis of endogenous testosterone. The nonsteroidal ligands are more favourable for clinical and therapeutic applications because of the lack of crossreactivity with other steroidal receptors (i.e. progesterone receptor, estrogen receptor, glucocorticoid receptor), which eliminates the unwanted feminizing side effects associated with steroidal antiandrogens. Moreover, they demonstrate a highly improved oral bioavailability as compared to their steroidal counterparts and are also open to various structural modifications due to their flexible backbone.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
The main chemical classes of non-steroidal antiandrogens available for clinical use are:
1) Propionanilide derivatives
2) Hydantoin derivatives
3) Phthalimide derivatives
4) Quinolinone derivatives
5) Arylisothiocyanato derivatives
6) Arylpiperazine derivatives
7) β-Alkylthioindolyl carbinols
8) 4-Ethoxycarbonylethyl curcumin analogs (ECECur)
9) Cyclocymopol derivatives
The class of non-steroidal molecules found to be most successful as antiandrogens were the propionanilide derivatives, the hydantoin analogues, the phthalimide derivatives and the indolyl carbinols. Of these, the propionanilide derivatives i.e. the bicalutamide and flutamide analogues and the hydantoin analogues i.e. the nilutamide analogues have shown the maximum potency.
1) Propionanilide derivatives: These are the first developed non-steroidal antiandrogens and include drugs such as flutamide (Eulexin), bicalutamide (Casodex) and hydroxyflutamide. Unlike the steroidal antiandrogens, these are pure antiandrogens (when bound to wild type i.e. WT AR). These are the most widely used drugs for the treatment of androgen-dependent prostate cancer and have good oral bioavailability. However, the clinical application of flutamide has been limited by its hepatotoxicity after long-term administration. Bicalutamide has replaced flutamide as the antiandrogen of choice for prostate cancer treatment since it has less hepatotoxicity and longer half-life (6 days in humans). With the presence of the chiral carbon in the structure, bicalutamide is administered as racemate. However, the in vivo antiandrogenic activity arises almost entirely from the R-isomer, which has approximately 30-fold greater binding affinity and is cleared at a rate 1/100th of the S-isomer.
The structure-activity relationship studies with this class of antiandrogens are presented below:
For flutamide analogues:
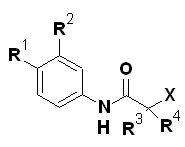
|
Compd. |
R1 |
R2 |
R3 |
R4 |
X |
ED50 (mg/kg/d) |
Agonism |
|
1a |
NO2 |
CF3 |
CH3 |
CH3 |
H |
0.5 |
NA |
|
2a |
NO2 |
CF3 |
CH3 |
CH3 |
OH |
0.5 |
NA |
|
3a |
NO2 |
H |
CH3 |
CH3 |
OH |
- |
NA |
|
4a |
NO2 |
H |
CH3 |
CF3 |
OH |
5.0 |
NA |
|
5a |
NO2 |
CF3 |
CH3 |
CF3 |
OH |
0.15 |
NA |
|
6a |
NO2 |
CF3 |
CH3 |
CHF2 |
OH |
0.12 |
NA |
|
7aa |
Cl |
Cl |
CH3 |
CH3 |
OH |
>10 |
NA |
a Structure 7a has –C=C- in place of –NH-C=O- bond.
For bicalutamide analogues:
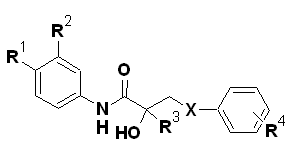
|
Compd. |
R1 |
R2 |
R3 |
R4 |
X |
ED50 (mg/kg/d) |
Agonism |
|
1b |
NO2 |
CF3 |
CF3 |
H |
S |
33% at 2.5 |
34 |
|
2b |
CN |
CF3 |
CF3 |
H |
S |
0.7 |
30 |
|
3b |
CN |
CF3 |
CF3 |
H |
SO |
0.3 |
|
|
4b |
CN |
CF3 |
CF3 |
H |
SO2 |
0.5 |
32 |
|
5b |
CN |
CF3 |
CH3 |
H |
S |
1.7 |
NA |
|
6b |
CN |
CF3 |
CH3 |
H |
SO |
1.4 |
NA |
|
7b |
CN |
CF3 |
CH3 |
H |
SO2 |
1.8 |
NA |
|
8b |
NO2 |
CF3 |
CH3 |
H |
SO2 |
1.5 |
NA |
|
9b |
CN |
CH3 |
CH3 |
H |
S |
50.0 |
|
|
10b |
NO2 |
CF3 |
CH3 |
4-Cl |
S |
1.6 |
NA |
|
11b |
NO2 |
CF3 |
CH3 |
3-Cl |
S |
18.0 |
NA |
|
12b |
NO2 |
CF3 |
CH3 |
2-Cl |
S |
5.0 |
|
|
13b |
NO2 |
CF3 |
CH3 |
4-F |
S |
1.1 |
NA |
|
14b |
NO2 |
CF3 |
CH3 |
4-F |
SO2 |
0.4 |
|
|
15b |
CN |
CF3 |
CH3 |
4-F |
S |
0.5 |
NA |
|
16b |
CN |
CF3 |
CH3 |
4-F |
SO2 |
0.5 |
NA |
From the SAR, it can be inferred that in the series, the most active compounds contained electron-withdrawing substituents in the aromatic ring and a branched alkyl chain α to the amide carbonyl. For flutamide, its 2-hydroxy derivative was the active metabolite. Compounds with two electron-withdrawing groups on the aromatic ring gave better potency than the one substituent. When the CF3 group was introduced at the C2 position of anilide having only one EWG, antiandrogenic activity of compound increased by five folds. Compound 6a where all the three factors were present showed the highest potency of the series. SAR revealed various factors which are responsible for antiandrogenic activity:
1. An electron-deficient aromatic ring separated from the tertiary carbinol by an amide link
2. A powerful hydrogen bond donor group
3. Fixed conformers involved in intramolecular hydrogen bonding
In addition, it was found that the biological profile was influenced greatly by the nature of the two other substituents attached to the carbon atom bearing the tertiary hydroxyl group.
In the bicalutamide series in particular, compounds with the cyano or nitro group at the 4’-position and the chloro or trifluoromethyl group at the 3’-position of the anilide ring gave improved antiandrogenic activity. In general, 2-trifluoromethyl compounds, showed a mixed agonist/antagonist activity. Antiandrogenic activities of the sulfide, sulfoxide and sulfonewere comparable in vivo and sulfones were the major metabolites of the sulfides in vivo. In the case of the arylthio analogues, parasubstitut









