About Authors:
Waghmare P.V.*, Chinchole A.S.1, Chavan D.V.2, Dr.Bhusnure O.G.3
Master of pharmacy, Department of Quality Assurance (*,1, 2,3)
Maharashtra College of Pharmacy, Nilanga, Dist. Latur (MS) 413521, India
*pradeep.waghmare90@gmail.com
Abstract:
A new generation of needle-free vaccine delivery devices (jet injectors) has been developed in an effort to decrease the risks of needle stick injuries to healthcare personnel and to prevent improper reuse of syringes and needles. Needle-free technology offers the very obvious benefit of reducing patient concern about the use of needle. Needle free injection gives very effective injections for a wide range of drugs and bioequivalent to syringe and needle, results in less pain, and is strongly preferred by patients. Additional benefits include very fast injection compared with conventional needles and no needle disposal issues. Not only it can benefit the pharmaceutical industry in increasing product sales, it has the added potential to increase compliance with dosage regimens and improved outcomes.
REFERENCE ID: PHARMATUTOR-ART-1888
Introduction:
Needle-free injection techniques can be used to administer vaccines and medications in the pork industry. Needle-free injection offers a fast, effective route of administration. There are hazards that must be addressed to safeguard employees who utilize needle-free injection systems; therefore, an enforced education program is crucial to the success of using needle-free injection in any pork operation. Needle-free injection technologies have been developed for injecting liquid formulations, as well as injecting drugs and vaccines in a solid dosage form. Needle-free injection systems are novel ways to introduce various medicines into patients without piercing the skin with a conventional needle. They can take the form of power sprays, edible products, inhalers, and skin patches. While hypodermic needles were first introduced during the 1800s, needle-free systems are relatively recent inventions. Today, they are a steadily developing technology that promises to make the administration of medicine more efficient and less painful.
Objective:
1. To review needle vs. needle-free injection systems and describe the different types of needle-free injection systems.
2. Less Painful and Potentially Safer
3. The key benefits of avoiding a needle and ease of use of a liquid jet injector do not outweigh the overall cost of goods compared with other delivery technologies.
4. Major advantages of needle-free systems are the elimination of broken needles, a more constant delivery of vaccines and drugs, and decreased worker safety risk.
Needle vs. Needle-free Injection:
1. Cost Efficiency
Needle-free injection systems can potentially reduce medical costs for the pork producer because the chance of injury to an employee from inadvertent needle sticks is eliminated. Needle-free systems also eliminate the purchase of needles.
Needle breaks, which can damage tissue and cause a decrease in overall yield and profitability, are also therefore eliminated.
However, the start-up costs associated with needle-free injection systems can be large. Pork producers should weigh the costs and benefits to these systems before adapting new technology.
2. Worker Safety:
Safety is a key ingredient to any pork operation. Employees must be properly trained on the use and maintenance of all equipment. Needle injection can be dangerous due to inadvertent needle sticks or cuts. However, needle-free injection is not 100% safe. Needle-free systems are designed for a high force dose to be administered very quickly and should only be used with proper training. These systems do offer a limited amount of risk to the operator, if properly trained, and exclude the possibility of needle sticks and cuts.
3. Sterility
Sterility is a key factor to proper vaccination and drug delivery. Sterility can be affected by human error. For example, the same needle may be used on multiple animals. Workers may forget to change needles when drawing vaccine from a bottle. Needle-free injection takes the needle out of the equation, and due to the high powered dosing mechanism, there is a little to no chance of cross contamination.
4. Pork Safety
The use of needles, along with human error, may also cause pork carcass defects. If needles are disposed of correctly or dropped after use there is always of a possibility of an animal ingesting the needle or being stuck in an unassuming place.
Needle-free injection systems eliminate residual needles and needle fragments from pork carcasses.1 The Pork Quality Assurance (PQA) Plus program recommends that all producers have a broken needle policy in place.2
5. Proper Dosage
Injection site is a crucial element in making sure that a proper dosage is received by the animal. A needle injection provides many unknown variables that can prevent proper dosing and in turn create havoc in your vaccination program.
Proper dosing is highly dependent on many factors. Among these factors are the size and age of the pig and the recommended route of administration. Different methods of administration such as subcutaneous (SQ) or intramuscular (IM) are very important in guaranteeing quality vaccination. If a vaccine or drug is not administered accordingly the effectiveness of the drug and the withdrawal time are altered. Incorrect injection sites in both needle and needle-free injection can impair pork safety.3
Needle although effective has several draw backs8:
1. Needles are expensive. The cost results in a lower vaccination rate, especially for children in developing countries.
2. Lack of reusability, if a needle syringe is not sterilized reusing it can lead to the spread of disease.
3. Many people have a fear of needles (often called Trypanophobia, Belonephobia or Aichmophobia) which causes them to avoid treatment. Needle pho-bia affects at least 10% of the general population.
4. Accidental needle sticks lead to injuries and possible infections.
Types of needle free injection systems:
Needle free technologies are of three types:
a) Powder injections
b) Liquid injections
c) Depot or projectile injection.
The Manufacturing Process:12, 13
There are numerous methods of producing each needle-free injection system. The following process focuses on the production of an air-forced system. These systems are made through a step by step procedure which involves molding the pieces, assembling them, and decorating and labeling the final product. The individual pieces are typically produced off-site and assembled by the needle free injection system manufacturer. All of the manufacturing is done under sterile conditions to prevent the spread of disease.
a) Making the pieces
- The first step requires the production of the component plastic pieces from plastic pellets. This is done by a process called injection molding. Pellets of plastic are put into a large holding bin on an injection molding machine. They are heated to make them flowable.
- The material is then passed through a hydraulically controlled screw. As the screw rotates, the plastic is directed through a nozzle which then injects it into a mold. The mold is made up of two metal halves that form the shape of the part when brought together. When the plastic is in the mold, it is held under pressure for a specified amount of time and then allowed to cool. As it cools, the plastic inside hardens.
- The mold pieces are separated and the plastic part falls out onto a conveyor. The mold then closes again and the process is repeated. After the plastic parts are ejected from the mold, they are manually inspected to ensure that no significantly damaged parts are used.

Figure 1.Parts of needle free injection
b) Assembling and labeling
The parts are next transported to an assembly line. In this production phase various events occur. Machines apply markings that show dose levels and force measurements. These machines are specially calibrated so each printing is made precisely. Depending on the complexity of the device, human workers or machines may assemble the devices. This involves inserting the various pieces into the main housing and attaching any buttons.
c) Packaging
After the assembly step, the injection devices are put into packaging. They are first wrapped in sterile films and then put into cardboard or plastic boxes. Each part is packaged so movement is minimal to prevent damage. For consumer products, an instruction manual is included along with safety information. These boxes are then stacked on pallets and shipped via truck to distributors.
How does it work?
a) Medication is driven at high speed through a tiny orifice
b) A fine stream of medication penetrates the tissue
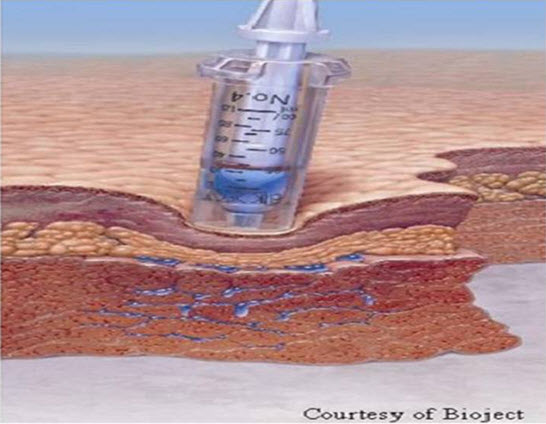
Figure 2: Injecting Medicament through Skin by Needle Free Injection
c) Injection event requires less than 0.5 seconds
d) Injections can be IM, SC or ID
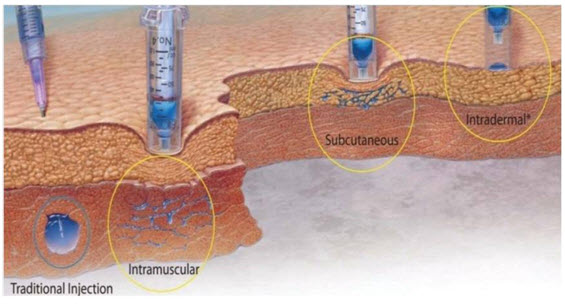
Figure 3: Types of Parenteral Route
Drug administration through conventional needle system and needle free injection technology. A spherical bolus is formed in case of conventional needle system where the surface area/volume ratio is very less when compared to needle free injected devices. Drug is dispersed as a spider web in case of needle free injected systems.
Mechanism of working: 9, 10
- Needle-free injection technology works by forcing liquid medication at high speed through a tiny orifice that is held against the skin. The diameter of the orifice is smaller than the diameter of a human hair. This creates an ultrafine stream of high-pressure fluid that penetrates the skin without using a needle.
- The design of the device has a major influence on the accuracy of subcutaneous delivery and the stresses imposed on the product to be delivered.
- The design must ensure that a sufficiently high pressure is generated to puncture the skin, while the subsequent pressure is reduced to ensure that the molecule is deposited comfortably at a level that does not reach the muscle tissue.
- High-pressure delivery could potentially damage fragile molecules, such as monoclonal antibodies.
- Successful delivery of such molecules, therefore, requires a device with carefully controlled power nuances. Several companies are involved in development of this technology, which includes, Antares Pharma Inc, Aradigm Corporation, Bioject Medical Technologies Inc and Biovalve Technologies Inc.

Needle free Injectors-Pulse:
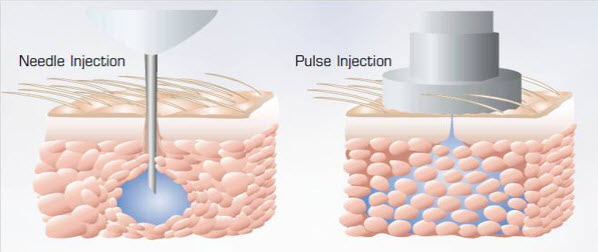
Needle free Injectors- Acushot:


Injection Methods:
Subcutaneous injections in small pigs should be given by pulling loose skin in the elbow or flank area. This technique is called tenting. In sows, the area just behind the ear is an acceptable sight for SQ injection. Intramuscular injection is conventionally administered in the neck just behind the ear. IM injection anywhere else is not acceptable because it will compromise pork safety and it should never be injected in the loin or ham muscles.
Types of Needle-free Injection Systems:
Needle-free injection systems are not a new development. The earliest systems were developed in the 1930s and have been used in a wide variety of medical areas over the years.4 Through innovation and technology there have been modifications and variations that allow for needle-free injection systems to be more widely available and effective to consumers.
1. Spring-load jet injector
The spring-loaded jet injector uses a spring mechanism that is drawn back. A trigger is then hit which release the spring creating a “jet stream” of vaccine or drug through the dermal layers of the skin. It is capable of subcutaneous, intramuscular or transdermal delivery. Each time the spring-load is activated the spring must then be manually redrawn to dose the next animal.
2. Battery-powered jet injector
The battery powered jet injector uses a small rechargeable battery pack to retract the dosing device. The dosing device has an electrical piston that is automatically redrawn after dosing. It is good for continuous use and minimizes worker fatigue. It is release by a small trigger. The injector resembles a battery powered hand drill. The battery powered system administers subcutaneous, intramuscular or transdermal dosage depending on the recommended method.
3. Gas-powered jet injector
This type of injecting system was one of the first developed. It uses an air/gas cartridge attached to the gun either directly or indirectly through a tubing system to deliver power to the injector piston. When the trigger is activated it releases the piston and creates a jet stream of vaccine or drug subcutaneously, intramuscularly or transdermally.
Problems it solves: 10
- Avoids needle stick hazard.
- No sharps disposal problems.
- Eliminates the concern for the re-use of needles.
- Injection pain is reduced in most cases.
- Speeds the injection cycle.
- Improved bio-availability of vaccines.
- Reduces the system cost of injection.
- Key Needle Free Manufacturers of the World
Advantages and disadvantages of needle-free injection devices (NFIDs) over needle-syringe devices in swine production:
Advantages:
1. Elimination of broken needles
2. Consistent vaccine delivery
3. Lower vaccine volume
4. Higher antigen dispersion
5. Elimination of worker needle sticks
6. Elimination of needle disposal
7. Less pain and stress
Disadvantages:
1. Higher start-up costs
2. Infrastructure for exhaustible gas systems
3. Higher requirement for training and maintenance
4. No one-size-fits-all NFID
5. Worker confidence in NFID
Implications:
- Advantages of needle-free vaccine delivery over conventional needle-syringe administration include elimination of broken needles, lower vaccine volume and greater antigen dispersion, elimination of accidental worker needle sticks, elimination of needle disposal, and less pain and stress.
- Adoption of NFIDs has been slow due to the cost of the unit and associated maintenance and gas infrastructure costs, greater complexity than needlesyringe devices, higher labor costs, and requirement for training.
- Immune responses to vaccines administered by NFID and needle-syringe technology are similar.
- Further studies under field conditions in commercial swine operations are needed to confirm the advantages of NFID vaccine delivery over conventional needle-and-syringe vaccine delivery.
Opportunities:
- Eliminate negative needle issues
- Probably reduce spread of infectious organisms
- Keep product supply cleaner
- Eliminate needle disposal problem
- Reduce pain and stress-both for animal and worker.
- Possibly reduce volume of product
- Consistent delivery of product
Marketed Product11
Several liquid jet injection technologies are available on the market including Ferring’s Zomajet 2 Vision, Merck Serono’s Saizen Cool. Click and Teva’s Tev?Tropin Tjet, which are all human growth hormone products. Zogenix’s Sumavel DosePro is also available, which is a sumatriptan injection for treating migraines. These liquid jet injection products, however, are expensive compared with most auto?injectors and pen?injectors, which also have the added advantage of being easily used by patients at home. The key benefits of avoiding a needle and ease of use of a liquid jet injector do not outweigh the overall cost of goods compared with other delivery technologies.
Some another Needle Free Injectors Available In the Market: 15-23
|
Technology/product name |
Company name |
Description |
|
Implaject |
Caretek Medical |
Simple, hand-held needle free injection device. Can be configured to be reusable with disposable cartridges. |
|
PowderJect |
PowderMed |
It painlessly delivers DNA vaccines to the skin in a dry formulation |
|
Zoma-jet 2 Vision |
Antares Pharma |
Customized version of Medi-jector vision licensed to Ferring for administration of their human growth hormone, Zomacton for distribution in Europe. |
|
Valeo (MJ8) |
Antares Pharma |
Next generation penstyle, spring-powered device. Designed for use with drugs in cartridge containers, rather than vials. |
|
Injex 30 |
Injex (HNS International |
Spring-powered handheld device with disposable ampoules that delivers 0.05-0.3 ml. Focused on insulin delivery. |
|
Intraject |
Weston medical |
Applicable to drugs including proteins, peptides, monoclonal antibodies, small molecules and vaccines |
|
Medi-Jector vision |
Antares Pharma, lnc |
It uses pressure to create a micro thin stream of insulin that penetrates the skin. |
|
Penjet |
Penjet Corporation |
A pre filled and disposable needle free injector |
|
Med-E- jet |
Evans enterprise |
Needle free injection |
|
Crossject |
Crossject |
Prefilled, single use disposable NFI. Uses chemical reaction to generate propellant at the time of administration |
Is the future needle-less? 11
Patients' want safer and better methods of taking their medicine. Additionally, with increased competition, pharmaceutical companies need new ways of differentiating their products and extending the patent life on proprietary drugs.
Many drugs and vaccines need to be administered parentrally, but there is limited scope for further new developments in the area of auto injectors and pen injectors. As such, there will always be demand for new technologies, such as needle-free injection devices. Although they are currently viewed as being costly which is one of the reasons the technology is not more widely used the price of liquid jet injectors will fall as use increases. They may also be able to offer other liquid formulation benefits such as improved stability or controlled release. Although these benefits can also be offered by needle based technologies, needle free devices will also offer the benefit of ease of use and no needle.
The biggest advances in needle free injection technologies will probably relate to their ability to inject solid dosage forms. The key challenge, however, will be the successful commercialization of the first solid dose injection product to prove that the key perceived risks of manufacturing scale up and regulatory approval for a novel drug delivery platform can be achieved.
Conclusion:
Needle-free injection systems have potential to improve efficiencies. Major advantages of needle-free systems are the elimination of broken needles, a more constant delivery of vaccines and drugs, and decreased worker safety risk. Needle free injection systems are customizable to each operation and can be modified to optimize productivity.
However, implementing a needle-free system can be challenging. Workers require training and education regarding any new technique. Start-up and training costs may also affect the interest in this technology for some pork producers.
Needle free injection technology offers effective injectors for a wide range of drugs & bioequivalent to needles and syringes. Needle free devices have demonstrated consistent delivery to the epidermis, the dermis, the subcutaneous and the intramuscular space. They offer less pain, avoid needle stick injuries and contamination, allows self administration and results in no needle phobia and are thus strongly preferred by the patients. Some of them are ideally suited to chronic injections of varying doses of insulin, proteins and monoclonal antibodies.
References
1.Houser TA, Sebranek JG, Bass TJ, Thacker BJ, Nilubol D, Thacker EL. Feasibility of transdermal, needleless injections for prevention of pork carcass defects. Meat Science. 2004:68(2); 329-332.
2.PQA Plus. Available at pork.org/filelibrary/PQAPlus/PQAPlusEd Book.pdf. Accessed September 21, 2011.
3.Sterle J. Providing a Safe, Wholesome Product: Administration of Medications to Ensure Pork Quality/Safety. 2009.
4.Chase CGL, Daniels CS, Garcia R, Needle-free injection technology in swine: Progress toward vaccine efficacy and pork quality. J Swine Health Prod. 2008; 16(5):254-261.
5.Gordon Moore, Moore Ag Safety-Needle-Free Injection Systems from fact sheet pork information gateway
6.Mason C., D.V.M. - from two field studies with Acushot and Pulse Needle-free injectors -2009-201
7. pharmtech.com/pharmtech/Drug+Delivery/Needle-Free Injection/Article Standard/Article/detail/707036
8.Sunitha Reddy M., Ranjith Kumar M., Sanjay Kumar K., Anil Goli, Santhosh Kumar P., Review on Needle free drug delivery systems, International Journal of Review in Life Sciences., 1(2), 2011, 76-82
9.http://www.pharmatutor.org/articles/needle-freeinjection- technology, Page=0,1
10.Rapolu Bharath Kumar Needle Free Injection Systems the Pharma Innovation vol. 1 no. 9 2012
11. pharmtech.com/pharmtech/Drug+Delivery/Needle-Free Injection /Article Standard /Article/detail/707036
12.Potera, C. "Making Needles Needless." Technology Review (September/October 1998): 67-70
13. answers.com/topic/needle-free-injection systems.
14.glidepharma.com/lead-drug-candidate-for-implaject.html.
15.In-pharmatechnologist.com/Materials-Formulation/PowderJect-renewed-in-Chiron-spin-out.
16.Antarespharma.com/products/
17.wikinvest.com/stock/Antares_Pharma_(AIS)/Development_Efforts_Mj8_Valeo_ Needle free_ Injection Systems
18.injex.org/?q=node/6
19.iptonline.com/articles/public/IPTFIVE100NP.pdf
20.mediject.com/about/overview.htm-
21.penjet.com/pages/pr_degas.html
22.cdc.gov/nip/dev/N¬3draft0007
23.crossject.com/
24.Mohanty C., Chandana P., Mannavathy D., Shrikanth D., Tabassum R. Needle free drug delivery system: A Review , international journal of pharmaceutical research and development 2011 Vol. 3(7): October 2011 (7-15)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









