 About Authors:
About Authors:
Kambham Venkateswarlu*, D.Z.Suhasini
Department of Pharmacology,
Sri Lakshmi Narasimha College of Pharmacy (JNTUA), Pallur,
Chittoor, Andhra Pradesh, India.
*k.v.reddy9441701016@gmail.com
ABSTRACT:
Lead optimization techniques are deals that new discovery is to choose the compounds with a known pharmacological action and proceed to modify the molecular structure of the compound systemically to get a drug with desired properties pharmacological action and pharmacokinetics. The compounds of the drugs are choosing for the study is called as lead.
Generally the identification and synthesis of compounds which are structurally related to the lead compound and testing their pharmacological activity. In the process it is possible to find a better drug than the lead compound.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1530
I. INTRODUCTION:
The discovery and development of a new drug is a complex process, full of hidden obstacles.
In order to respond to the great variety of therapeutic needs and meet national and international regulatory requirements, these processes, which themselves demand an integrated multi disciplinary approach, must be conducted with absolute scientific rigor.
The discovery and development of a new drug can take between 7 and 15 years and experts estimate the average cost to be $us802m (2000).
The structure of the lead compound is modified by synthesizing the analogue, to amplify the desired activity and to minimize or eliminate the unwanted properties.
II.BASIC PRINCIPLE:
The basic principles in the discovery and development of a drug may be summarized as follows:
2.1 Discovery:
The discovery stage can last up to 6 years and experts estimate its average cost to be US335m (2000).
The proteins produced by transcription of our genes ensure that our body’s main biological functions are carried out.
A faulty gene or protein is often what lies behind a disease.
To treat a particular disease, it is first necessary to identify the biological targets (i.e., a protein or other biopolymer) known to be involved in this disease’s etiology and then discover the compound or compounds that have an effective and specific therapeutic capability and a minimum number of side effects.
Specialists in the biological-sciences and medicinal chemistry work in close collaboration throughout the entire process of drug discovery.
2.3 Development:
The development of the drug can take as long as a decade at an average cost estimated at $us467m (2000).
Once the compound or compounds have been chosen, they must be transformed into a drug this process involves several series of trials on animals and humans, all intended to ensure that the drug may be administered to humans with minimum possible risk and that it is superior to or otherwise complements existing drugs with the same therapeutic function.
These trials are subject to the rigorous controls required by the regulatory authorities such as Health-Canada or the US Food and Drug Administration (FDA).
In addition to specialists in biology and therapeutic chemistry, the discovery of a new drug involves the collaboration of pharmaceutical R&D specialists and clinical research teams, composed of doctors, nurses and other health specialists.
2.4 The Discovery and Development of a New Drug over View:
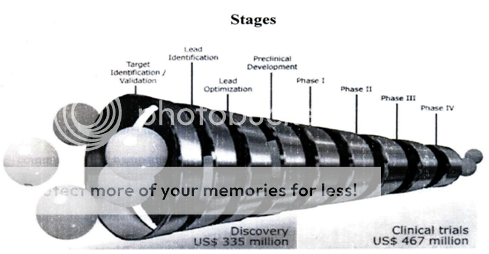
Experts estimate the cost of the development of a new drug to be $us802m (2000). This amount includes expenses related to the interrupted development of molecules that failed to meet specific characteristics required for commercialization. It also includes the cost of capital investments.
III. TARGET IDENTIFICATION / VALIDATION:
Duration: From several months to several years
In order to ensure the successful development of new drugs the pharmaceutical industry requires considerable scientific and financial resources must from the strategic alliances with industrial pertness the university research community and companies conducting research under contract drugs that act in new ways upon biological targets specific to the diseases requiring new therapeutic approaches.
Thus identification of therapeutic targets requires knowledge of diseases etiology and the biological systems are associated with it.
Recent findings issuing from the “human genome” project have seen the odyssey of drug discovery become very sophisticated indeed. Over the last 50 years most of the drugs commercialized were developed for around 500 known biological targets now, the many projects involved in the study of the “genome and proteome” of humans and other organisms are beginning to contribute to the discovery of new biologically interesting targets.
IV. LEAD IDENTIFICATION:
Between 5 and 50000 compounds \are examined in the laboratory, of which only 100 to 200 are perfected in order to be tested on systems in vitro and in vivo.
Once the therapeutic target has been identified, scientists must then find one or more leads (e.g., chemical compounds or molecules) that interact with the therapeutic target so as to induce the desired therapeutic effects, e.g., through antiviral or antibacterial activity.
In order to discover the compounds whose pharmacological properties are likely to have the required therapeutic effects, researchers must test a large variety of them on one or more targets.
First of all, biologists ensure that the chosen compounds have the desired therapeutic or antiviral effects on the target. Then, they test the compounds relative toxicity or in the case of vaccine, their viral activity using in vitro cellular and/or tissue systems. Finally, they check their bioavailability in vivo on animals.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
V. LEAD OPTIMIZATION:
Duration:From 4 to 6 months
The 100 to 200 chosen compounds are examined in the laboratory in order to perfect their physiochemical properties, their pharmacokinetic behavior and the therapeutic effectiveness. Around twenty (20) will be selected to be tested on.
The purpose of this stage is to optimize the molecules or compounds that demonstrate the potential to be transformed into drugs, retaining only a small number of them for the next stages.
To optimize these molecules, scientists use very advanced technique. For example, using X-ray crystallography and in silico (computer) modeling, they study how the selected molecules link themselves to the therapeutic target, for example, a protein or an enzyme. These data allow the medical chemists to modify the structure of the selected molecules or compounds, if necessary, by screening, thereby creating structural analogues.
VI. PRE – CLINICAL TRIALS:
Duration: A Minimum of 4 To 6 Months
The twenty or so perfected compounds are evaluated both in vitro and in vivo. Of which between one and five will have the characteristics required for Phase-I clinical studies.
The development potential of a candidate molecule depends essentially on its capacity to be administered to humans and show therapeutic effectiveness with an acceptable level of side effects.
The regulatory authorities require pharmaceutical companies to demonstrate the safety of the drug for humans and to prove that the therapeutic advantages of the compound greatly outweighs any associated undesirable side effects such as migraine or high blood pressure in the case of cancer treatment.
Chemist, Biochemists, Pharmacologists, toxicologists and Histologists continue to evaluate the pharmacokinetic, pharmacodynamic and toxicological properties of the compound in vitro and in vivo (on animals).
Chemists and Pharmacists develop different dosages and pharmaceutical formulations taking particular account of the physic-chemical and metabolic properties of the molecule and the biomedical characteristics of the targeted therapeutic application. For example, for pathologies affecting young children, it may be preferable to use a syrup, which is more easily swallowed than a tablet.
VI. PHASE –I:
Duration: Around 18 Months
These trials are carried out using a small number of healthy subjects, generally between 100 and 200 people divided into small groups of 10 to 20 subjects.
These tests are carried out with the help of the most promising compounds (between one and five). Generally, between 1 and 3 molecules will be selected for phase H.
The aim of the first clinical trials (Phase I) on human is to help evaluate and understand the behavior of the molecule or compound for the first time on humans. Thus, they acquire deeper knowledge of several parameters including, for example:
* The impact of the molecule on the organism (metabolism, bone tissue, blood, liver, brain activity etc) various secondary effects etc.
* The different reactions to the drug of men and women.
* The interaction between food and the absorption of the drug. The subjects are subjected to increasing doses so as to determine both the minimal dose at which side effects can be detected and the maximum dose tolerated.
VII. PHASE II:
Duration: 12 to 24 Months
The compounds selected are tested on 100 to 500 patients. At the end of these trials, 1 or 2 molecules will be phase III. This phase lasts between 12 and 24 months.
The Phase II clinical trials aim to check the harmlessness or degree of effectiveness of those (1 to 3) promising compounds that have successfully completed the previous stages.
Phase II clinical trials are carried out on 100 to 500 patients suffering from the target disease. The patients are divided into subgroup so that different tests may be conducted in parallel fashion.
This phase allows:
* Study of the side effects and risks associated with shirt-term use.
* Analysis of the compound’s impact on metabolism.
* Optimization of the dosage and treatment duration.
VIII. PHASE- III:
Duration: May Last Several Years
The lead molecules are administered to a large number of patients (1,000 to 8,000), coming from various regions and divided into independent groups.
Clinical trials in Phase III have several objectives, for example:
* Proving the therapeutic effectiveness of the compound under realistic usage conditions.
* Demonstrating that the compound is as effective drugs or forms of treatment, if not more so.
* Rigorously comparing the patient groups treated with the candidate drug with the control groups to whom a placebo has been given.
* Testing different methods of administering the drug (tablet, syrup, inhaler etc.)
These trials, which are conducted along with other tests on rodents, allow for even more precise understanding of the drug’s safety profile, the occurrence of undesirable side effects and long term toxicity. These are all documented in a manner satisfactory to the regulatory authorities.
IX. PHASE IV:
Duration: Several Years
These studies analyze the reactions of million of patients around the world.
During this phase, research is mainly aimed at:
* Spotting possible undesirable side effects associated with long-term use by a large number of patients.
* Extending use of the drug to different classes of patients such as children.
* Finding new therapeutic opportunities or new formulations that may increase the effectiveness of the drug and allow it to be administered to a larger number of patients.
* Demonstrating that the benefits of requirements justify the reimbursement of the cost of the drug by public and private health insurance programs.
These studies are in part required by the regulatory authorities.
X. HIT TO LEAD OPTIMIZATION:
10.1 Drug Metabolism:
There is a growing need for education in drug metabolism within the pharmaceutical industry that is not being met at the University level often such as skills are being taught on the job in pharmaceutical companies. In response to this need we offer an intensive half-day short course to provide you with the fundamental skills needed in drug metabolism and deposition for drug discovery. This course will cover the most recent technology and techniques in drug metabolism.
10.2 Cardiovascular inflammation:
From basic concept to clinical relevance organized by zoria galis (Lilly Research Laboratory) and Douglas Vaughan (Vanderbilt University), the Symposium will address new evidence suggesting that an inflammatory state is a common pathological mechanism for cardiovascular diseases and related conditions including metabolic syndrome and obesity. The hypothesis that inflammation is a driver of acute cardiovascular events and its relation with oxidative stress will be presented from a basic science and clinical perspective. Presenters will speak on the promise of using markers of inflammation and the potential of targeting inflammation-related pathways for diagnosing, preventing and treating cardiovascular diseases.
10.3 New Strategies for Lead Optimizing of Novel Anti-Inflammatory Agents:
Organized by Jerry Skotnicki (Wyeth Research) and Shripad Bhagwat (Ambit Biosciences), Lead optimization to identify drug candidates involves optimization of multiple parameters such as potency at the site of action, cell penetration, solubility, oral absorption, metabolic stability, elimination, efficacy and safety. While the science behind and understanding of optimization of some of these parameters is relatively well understood, the others are more empirical. A variety of novel techniques are devised that enable the medicinal chemists to tackle issues in lead optimization. This medicinal chemistry symposium will focus on the discovery of anti-inflammatory drugs using novel techniques for addressing lead optimization issues.
10.4 New Drugs:
Targeting inflammation in disease organized by Doug Morgan (Biogenldec) and Bernhardt Zeiher (Pfizer Global R&D). in this symposium we will focus on therapeutic agents targeting a wide array of diseases to emphasize the reality that inflammation spans all organs and disease states through common pathway and processes. The goal of this session is to expand our perspective of novel drug discovery to include the practical aspects of achieving “First Time in Human” clinical studies (Phase I) and designing Phase II studies to provide “Proof of Concept in Humans.” Each talk is to appeal to the broad audience of Biochemists, Pharmacologists, Medicinal Chemists and clinical colleagues.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
XI. THE LEAD OPTIMIZATION TECHNIQUES:
11.1 Computer Aided Drug Discovery:
Our computer aided `drug discovery group helps to discover new chemical leads and optimize leads towards preclinical development.
In cotemporary drug discovery, there are three overlapping phases: hit-identification, hit-to-lead and lead optimization. Hit-identification may involve high-throughput screening of a biological target or computer-aided selection of compounds for testing against the target. After initial biological testing, the verified hits often undergo hit-to-lead analysis in order to progress a few sets of compounds that are expected to develop into a lead series and can then be progressed via lead optimization into a few preclinical candidates.
Our CADD term contributes at each of these stages by performing computer calculations to aid in the understanding and prediction of the many biological, biochemical and physicochemical properties of chemical compounds that are monitored during a project.
11.2 Protein Structure Based Tools:
Some drug discovery projects have three-dimensional information of the protein target available. We use protein structure prediction tools, such as homology modeling, in concern with molecular simulations to refine, identify and understanding the binding site. Typically, structure-based design and docking techniques are used to aid in either lead identification or lead optimization. In lead optimization, we often use docking and simulation techniques to identify areas of molecules that can be improved to increase potency and areas that may be charged to improve physical or ADMET properties without decreasing potency.
11.3 Commercial Sample Collection:
Our commercial sample collection library contains 124,560 drugs like compounds selected from a database of 2.5 million unique, commercially available chemical structures using our unique computational algorithms.
The 2.5 million compounds were filtered by first selecting for drug-like and ADME characteristics and then for chemical diversity utilizing our novel median partitioning algorithm. The compounds were then rigorously analyzed for purity and correct structural assignment using LC-MS and where necessary, NMR, finally, to complete the balance of the collection, a set of 25,000 compounds was handpicked by our senior medicinal chemists from pre-qualified vendors for optimum lead seeking, drug-like properties.
Parallel synthesis chemistry is an important tools in generating new drug leads. Using cutting-edge robotics and miniaturization techniques, our scientists create small molecule “libraries” that can include hundreds or thousands of compounds directed at one drug target. These libraries may then be screened against drug targets or used as building blocks to produce more focused libraries. We also provide custom synthesis of scaffolds, building blocks and novel reagents. Using parallel synthesis technique, we can rapidly produce thousands of analogs around a screening hit.
11.4 Overview of Services:
* Synthesis of both large diversity libraries and focused (SAR) libraries.
* Bulk scaffold synthesis and resin production.
* State of the art automated, semi-automated and manual production methods.
* Proprietary technology, research and development capabilities.
* Commercial and in-house database management software.
* Resynthesis of single compounds identified as “hits” on larger scale and at higher purity.
11.5 Cheminformatics & Ligand-Based Tools:
When no suitable protein structure is available, we use chemical similarity and pharmacophore analysis. Using 2D and 3D methods, we can search for new synthetic modifications.
We have developed computational methods to capture similarities of molecules and we use these approaches to search database for molecules with biological activity similar to query compounds. In addition, our compound partitioning techniques explore “active neighborhoods” of hits obtained from screening. These techniques are particularly useful in exploring the structure-activity relationship around a set of hits and in identifying new chemical classes as new leads.
Moreover, we maintain a large database of compounds from many properties and commercial databases. This set of more than 5 million compounds is searchable by any of our already existing virtual screening methods. Based on analysis by our synthetic chemists, we have accumulated a large number of scaffolds that are amenable to parallel chemistry and are computer searchable.
11.6 Lead Identification & Optimization:
We conduct database mining and virtual screening projects, in particular, we identify compounds with activity similar to screening hits,3D-pharmacophore searches or by docking. In collaboration with our medicinal chemistry teams, we develop QSAR, pharmacophore and structure-based design techniques to accelerate lead-optimization. Along with optimization of potency, we provide support in prediction of ADME and physical properties.
11.7 Structure Based Design & Virtual Screening:
To guide “hit-to-lead” chemistry programs, we use computational docking techniques to screen compound collections on binding sites and explore binding characteristics of hits. We carry out virtual screening projects of databases consists of several hundred molecules using three dimensional structures of targets, if available, and/or hits from screening as templates, using site-specific scoring methods, we provide binding-site models for analog design.
11.8 Design of Focused Libraries:
In collaboration with synthetic chemistry teams, we have designed compounds libraries that are focused on specific activities, specific therapeutic targets or target areas. We provide support for ongoing or new chemistry programs in these areas by identifying preferred core structures or chernotypes for diversification. In addition, we assemble focused libraries by subset selection from proprietary or commercially available compound sources.
11.9 Metabolism & Bio-Transformation:
Our bio-catalysis technologies provide valuable tools for drug discovery and development, completing and enhancing our core capabilities in chemical synthesis. With expertise in synthetic bio-catalysis, fermentation, molecular biology, enzymology, and process engineering, we can exploit biosynthetic methods to develop a new route to a target compounds, a single step in a synthetic route or anything in between.
11.10 Biotransformation:
Bioprocess such as organic phase bio-catalysis and fermentation offer many benefits when integrated with traditional synthetic chemistry including more efficient, economical routes to target compounds. The ability to produce molecules that are difficult to make using traditional organic synthesis, region and stereo-selective transformations, moderate reaction conditions, less side products and solvent waste.
11.11 Large Scale Bio Catalytic Synthesis:
Additionally we have developed many classes of biocatalysts for large scale synthesis, supported by in-house physical characterization, method development and separation. The diversity of microbial and enzyme catalysts in our collection increases the breadth of transformations. We excel at designing bio-catalytic processes that yield a more efficient route to the desired molecule and fit into a commercially viable, economic process.
Our integrated approach uses chemistry and biology in tandem to develop a better compound, improve a process and reduce R&D costs.
11.12 In Vitro Metabolism:
Our metabolic screening technologies help identify compounds with maximum metabolic stability, minimize the potential for drug-drug interactions, and reduce late-stage failures. When used in tandem with our chemical synthesis and bioscience capabilities, these technologies help prioritize compounds for optimization by identifying structural characteristics that favor a desirable metabolic profile and providing important data about metabolic properties and enzyme interactions of compounds.
11.13 Metabolic Stability Assays:
These assays evaluate the expected “first pass” stability of a lead compound using incubation with liver microsomes and liepatocytes from human and animal species. Our assays help assess primary metabolism and pharmacokinetics in the liver, enabling customers to identify early in the process if a compound is chemically stable within the body and metabolized at a pharmacologically acceptable rate. These characteristics can be considered with structure activity relationships in a lead optimization program.
11.14 Metabolic Profiling Assays & Metabolic Production:
These assays identify metabolite produced from the interaction of a compound with metabolic enzymes. Our testing protocol includes incubation of the lead compound with liver microsomes, hepatocytes and isolated human CY450 isoforms to determine the relative amounts of metabolites. Our expertise with enzymology and parallel, high throughput synthetic biocatalysis enables us to rapidly assess the impact of many classes of metabolic enzymes affecting drug clearance and pharmacokinetics. We can identify and scale-up metabolites using traditional chemical synthesis or biocatalysis in order to study biological activity, toxicity, side effects, structural confirmation or further modify metabolites through medicinal chemistry and bio catalysis.
11.15 Cyp-450 Iso Form Analysis:
Cytochrome P-450 (CYP-450) isoforms are an important class of metabolic enzyme that act on chemical compounds as they enter the human body. In order to identify specific CYP-450 isoform interactions with a test compound, we use liver fractions and recombinant versions of individual CYP-450 isoforms. This analysis uncovers possible drug-drug interactions, the metabolic path of the compound, the involvement of CYP-450 isoforms in generating specific metabolites and provides information for further studies of kinetics, clinically relevant polymorphisms and other information.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
11.16 Cyp-450 Inhibition Assay:
Test drug compounds may inhibit the activity of a specific CYP-450 isoform causing changes to the body’s metabolism that can lead compounds using several different human CYP-450 isoforms to anticipate these potential side effects. Using IC50 values and Ki values for inhibition of CYP-450 isoforms, we measure the potential that a test compound will significantly inhibit a particular CYP-450 isoforms, as well as the concentration at which the compound is likely to inhibit a CYP-450 isoform.
11.17 Biocatalysts:
Our proprietary combinatorial biocatalysts technology platform is a powerful tool for generating focused libraries of derivatives, analogues, and possible metabolites from existing lead compounds.
Our technology uses iterative enzymatic and microbial reactions to create libraries of derivatives from lead compounds, both small molecules and complex natural products using this unique technology. We can quickly create diverse variations of promising leads for a broader understanding of structure reactivity relationships, expand patent coverage or introduce novel diversity in a library of compounds. Moreover, biocatalysis has demonstrated advantages that stem from the inherent chemo-, region- and stereo selectivity of enzymes, allowing us to make structural modifications that are difficult or impractical to achieve solely by chemical synthesis.
Our on-site analytical chemistry department helps us ensure that we meet your required purity label and provides compound identification services.
11.18 Fermentation:
Our fermentation group has developed many processes for a wide range of compounds, meeting aggressive timeframes and/or economic viability criteria.
In many cases, research process begins at milliliter scale in shake flasks and parallel mini fermentors, which are used to discover strains most suitable for a desired secondary or biotransformation product and to conduct initial process optimization. We can culture process samples and conduct sophisticated GC, HPLC, LC-MS, LC/MS/MS analysis on several hundred samples simultaneously. NMR analysis is also readily available for smaller numbers of samples. Extensive scale up experience and development vessels allow rapid progress to the liter and then to 10s to 100s of liters of fermentation. We maintain a bank of highly flexible and computer controlled 20-L scale fermentors that can be operated and monitored in a multitude of different configurations.
11.19 Discovery Analytical Services:
Analytical services are critical at every stage of pharmaceutical R&D, from the first synthetic step of even the shortest process, all the way through manufacturing.
We provide broad analytical support for drug discovery, preclinical, clinical development, and manufacturing. Strong problem-solving capabilities make us uniquely suited to take on the most formidable challenges posed in new small molecule drug research. With years of industry experience, our analytical scientists understand pharmaceutical chemistry. State of the art technologies and instrumentation along with close collaboration with synthetic organic chemists ensure that our analytical team asks the right questions, and then uses the right tool to solve even the most difficult problem.
To support early stage discovery, we provide the following analytical services:
11.20 Chromatographic Assay Development:
The foundation of any analytical development team is the chromatographic development group. Our experienced team of analysts can reliably develop and validate stability indicating and impurity assays for active pharmaceutical ingredients and drug products.
Our state of the art facilities and instrumentation includes dozens of HPLCs (including UV/PDA/ELSD/RI/Fluorescence detection) and multiple GCs (including FID, ECD, and NPD). We have particular expertise in APT/impurity assays and drug product assay development.
11.21 Use of Optimization Technique:
No algorithm for optimizing general nonlinear functions exists that will always find the global optimum for a general nonlinear minimization problem in a reasonable amount of time. Since no single optimization technique is invariably superior to others, PROC CALIS will find the correct solution. All optimization techniques in PRO CALIS use O (n2) memory except the conjugate gradient methods, which use only O(n) of memory and are designed to optimize problems with many parameters.
The PROC CALIS statement NLOPTIONS can be especially helpful for tuning applications with nonlinear equality and inequality constraints on the parameter estimates. Some of the options available in NLOPTIONS may also be invoked as PRO CALIS option. The NLOPTIONS statement can specify almost the same options as the SA S/OR N LP procedure.
Nonlinear optimization requires the repeated computation of
* The funct ion values (optimization criterion).
* The gradient vector (first order partial derivatives).
* For some techniques, the (approximate) Hessian matrix (second-order partial derivative).
* Values of linear and nonlinear constraints the first-order partial derivatives (Jacobean) of nonlinear constraints.
For the criteria used by PROC CALIS, computing the gradient takes more computer time than computing the function value and computing the Hessian takes much more computer time and memory than computing the gradient, especially when there many parameters to estimate.
Unfortunately optimization techniques that do not use the Hessian usually require many more iterations than techniques that do use the (approximate) Hessian and so they are often slower. Techniques that do not use the Hessian also tend to be less reliable (for example, they may terminate at local rather than global optima).
ACKNOWLEDGEMENT:
1] Thanks to the principal of Sri Lakshmi Narasimha College of Pharmacy, Prof. S.RAMADOSS.
2] Heartily thanks to the Asst.Prof. A.SHANTHI, Department of Pharmacognosy.
3] special thanks to the following Asst.Prof.V.SHIVAKUMAR.
Asst.Prof. J.THIRUMARAN
Asst.Prof. G.MARIYAPPAN
BIBLIOGRAPHY:
1. Anderson VL, MacLean RA. Design of experiments: A realistic approach. Marcel Dekker Inc., New York, 1974.
2. Augsburger LL, et al. An approach toward establishing a scientific foundation for interpreting regulations and workshop reports on scale-up and post-approval changes. Pharm Res, 11 (): S 161, 1994.
3. Avallone HL. Development and scale-up of pharmaceuticals. Pharm Eng, 10 (4):3 8-1 1, 1990.
4. Baert L, Fanara D, Remon JP. Crrelation of extrution forces, raw materials and sphere characteristics. Int J. Pharm, 44:676-678, 1992.
5. Berman J, Schoeneman A, Shelton JT. Unit dfose sampling: a tale of two thieves. Drug Dev Ind Pharm, 22(11):1121-1132, 1996.
6. Berry H, Ridout CW, J Pharm Pharmcol, 2:619, 1950.
7. Bohindar NR, et al. selecting key parameters in pharmaceutical formulations by principal component analysis. J Pharm Sci, 64:966, 1975.
8. Bolhuis GK, Lerk CF. Pharm. Weekblad, 108:469, 1974.
9. Bolton S. flawed statistical analysis and representations of fact. Drug Dev Ind Pharm, 231(8):851-853, 1997.
10. Box GEP, Draper NR. Evolutionary Operation. Wiley, New York, 1969.
11. Box GEP et al. statistics for experimenters. Wiley, New York, 1978.
12. Brittain HG et al. physical characterization of pharmaceutical solids. Pharm Res, 8:963, 1991.
13. Buckingham E. on physically similar systems; illustrations of the use of dimensional equations. Phys Rev NY, 4:345-376, 1914.
14. Chamberlain R. computer systems validation for the pharmaceutical and medical device industries. Alaren, Libertyville, IL, 1991.
15. Chang RK, Lai JW. Analysis of content uniformity data using a scatter plot and the tolerance ellipse. Pharm Tech, 5:60-66, 1994.
16. Chapman KG. a history of validation in the united states.
17. Chew V. experimental design in industry. Wiley, New York, 1958.
18. Chowhan ZT. Development of a new drug substance into a compacted tablet. Pharm Tech, 9:58-67, 1992.
19. Cochelard E et al. development of new software for an interactive analysis of particl size. Drug Dev Ind Pharm, 22()721-730, 1996.
20. Darvas F. application of sequential simplex method in designing drug analogs. J Med Chem, 17:799, 1974.
21. Davies OL. The design and Analysis of Industrial Experiments. Longman Group Ltd., New York, 1978.
22. Dobberstein RH et al. computer-assisted experimentsl design in pharmaceutical formulation. Pharm Tech, 3:84-94, 1994.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









