About Author:
Alex Martin
Department of Pharmaceutical Chemistry,
St. Joseph’s College of Pharmacy
University of Health Sciences, Cherthala-688524 (Kerala), India.
aalexmartin@rediffmail.com
Abstract
Oxadiazole is a five membered heterocyclic ring which is a versatile lead compound for designing potent bioactive agents. It exists in four isomeric forms. One of its four isomers 1,3,4-oxadiazole exhibited a wide range of biological activities which includes anti-bacterial, anti-tubercular, anticonvulsant, hypoglycemic, anti-allergic, enzyme inhibitor, vasodilatory, antifungal, cytotoxic, anti-inflammatory, analgesic, hypolipidemic, anticancer, insecticidal activities etc. The present review has basic information about 1,3,4-oxadiazole and its biological activities.
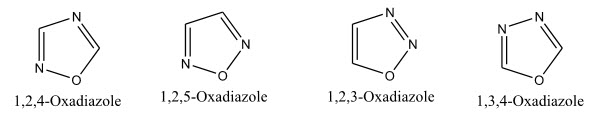
Oxadiazoles is a heterocyclic ring and is considered to be derived from furan by the replacement of two methane (-CH=) groups by two nitrogen (-N=) atoms. There are four possible isomers of Oxadiazole, depending on the positions of hetero atoms and they are named as 1,2,3; 1,2,4; 1,2,5; 1,3,4-oxadiazoles. 1,2,4-Oxadiazole, 1,2,5-Oxadiazole, and 1,3,4-Oxadiazole are known, but the 1,2,3-isomer is unstable and reverts to the diazoketone tautomer. The stable oxadiazoles appear in a variety of pharmaceutical drugs including raltegravir, butalamine, fasiplon, oxolamine, and pleconaril.
REFERENCE ID: PHARMATUTOR-ART-1906
Literature review reveals that the 1,3,4-oxadiazole undergo a number of reactions such as Electrophilic substitution, Nucleophilic substitution, Thermal and Photochemical. This has been exploited in the preparation of 1,3,4-oxadiazole therapeutic molecules for various applications. In view of this, an attempt has been made to review the biological activities of 1,3,4-oxadiazole.
Antimicrobial activity
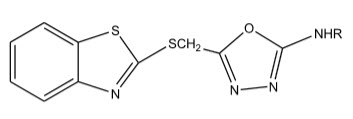
Radha et al 1synthesized 5-(benzothiazol-2-yl-thiomethyl)-1,3,4-oxadizole from mercaptobenzothiazole which showed moderate anti-bacterial and anti-inflammatory activities.
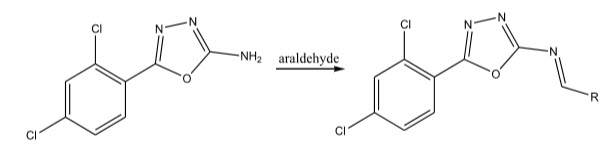
The bactericidal activity of 1,3,4-oxadiazoles was screened by Kataky et al 2 by the synthesis of 2-amino-5-(2,4-dichlorophenyl)-1,3,4-oxadiazoles. This will give 1,3,4-oxadiazole-2-iminobenzylidine with appropriate araldehydes. The products exhibited good anti-bacterial activity.
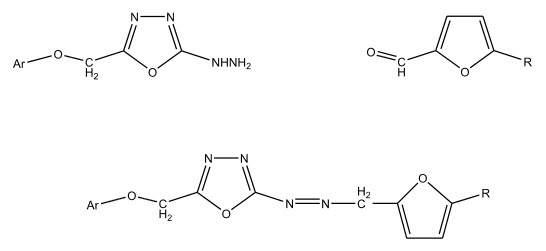
Various 5-substituted-1,3,4-oxadiazol-2-hydrazides were synthesized by Kalluraya B. et al 3 by the reaction of oxadiazole with hydrazine hydrate. The oxadiazole hydrazide when condensed with 5-substituted-2-furfural gave hydrazones. These compounds were found to be active against gram positive and gram negative bacteria.
2-(5’-thioxo-1,3,4-oxadiazolin-2-yl) indoles were synthesized by Sonar VN et al 4 by the reaction of indole-2-carbohydrazides with CS2 and KOH. Screening of these compounds has shown that they posses moderate activity against S. aureus, E. coli, P. vulgaris and B. subtilis.
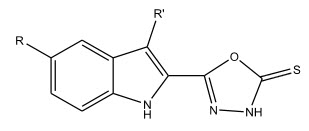
Nailesh Joshi et al 5 synthesized some 2,5-disubstituted-1,3,4-oxadiazoles . The compounds have been tested for their anti-microbial and anti-fungal activity.
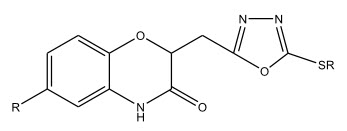
2-(3,4-dihydro-3-2H-1,4-benzoxazin-2-yl)methyl-5-(alkyl/arylthio)-1,3,4-oxadiazoles have been synthesized by Y. Jayamma et al 6 and screened for their anti-microbial activity.
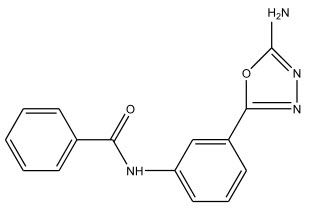
The synthesis of 2-arylamino-5-(p-nicotinamidophenyl)-1,3,4-oxadiazoles was carried out by Vimal R. Shah et al 7 from ethyl p-nicotinamidobenzoate which in turn was obtained by the treatment of nicotinic acid with thionyl chloride followed by the reaction with ethyl p-aminobenzoate in pyridine. The anti-bacterial activity of the compound was determined by the cup plate method.
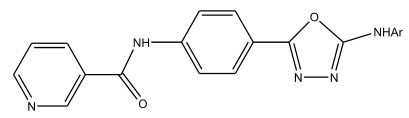
A series of 1,3,4-oxadiazoles were prepared by Kapoor et al 8 by the cyclisation of respective hydrazides. They were screened for their anti-bacterial, anti-fungal and anti-mycobacterial activities by agar well diffusion method.
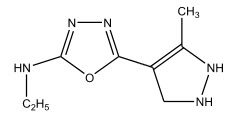
Antitubercular activity
Chaudary B.R et al 9 synthesized 1,4-benzothiazinyl-1,3,4-oxadiazoles by the condensation of 1,4-benzothiazinylthiosemicarbazides in the presence of an alkali. The compounds exhibited anti-tubercular activity.
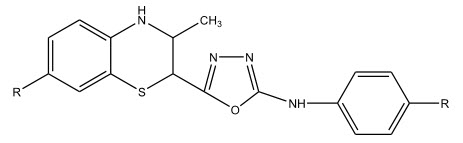
Parekh et al 10 synthesized 2,5-disubstituted-1,3,4-oxadiazoles and studied their anti-microbial activity against several microbes and anti-tubercular activity against Mycobacterium tuberculosis.
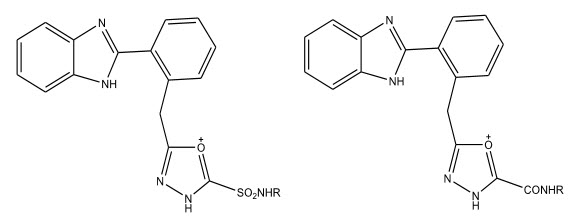
A novel series of 2-(substituted phenyl)amino-5-[4-(4-methoxybenzoylamino)phenyl]-1,3,4- oxadiazoles were synthesized and were evaluated for their anti-tubercular activity by Kucukguzel SG et al 11 . The compounds were found to possess moderate anti-tubercular activity.
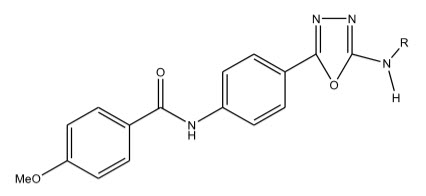
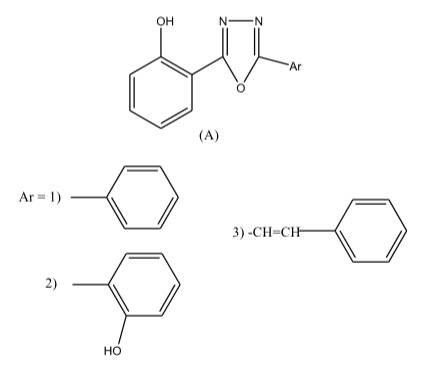
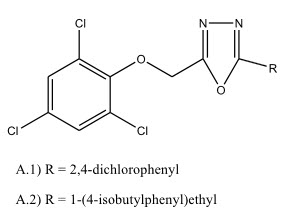
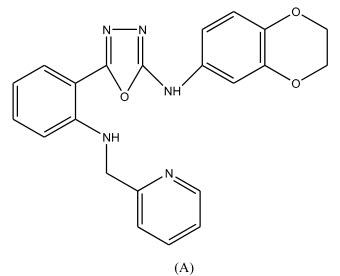
Some novel 1,3,4-oxadiazole derivatives have been synthesized by Pattan R. Shashikant et al 12 and the compounds were evaluated for their anti-tubercular activity against H37RV strain and compared to the standard drug Streptomycin. Compound A.1) have shown promising activity and compound A.2) and A.3) have shown moderate activity.
Anti-inflammatory activity
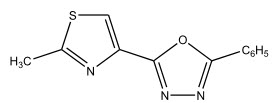
S.N. Sawhney et al 13 reported the synthesis of twenty compounds belongings to five different series namely, 2-(2-methylthiazol-4-yl)-5-aryl-1,3,4-oxadiazoles, 2-(2-methylthiazol-4-yl)-5-substituted-amino-1,3,4-thiadiazoles and 5-(2-methylthiazol-4-yl)-3-mercapto-4-substituted-4H-1,3,4-oxadiazoles as potential anti-inflammatory agents.
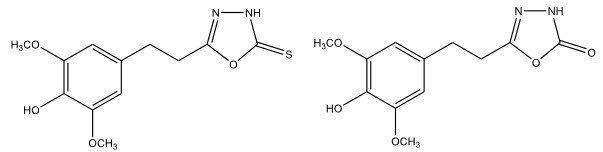
A series of 5-[2-(3,5-dimethoxy-4-hydroxyphenyl)ethyl]-1,3,4-oxadaiazoles were synthesized by Mullican H.D et al 14 with the aim of discovering dual inhibitors of 5-lipooxygenase and cyclooxygenase with improved pharmacokinetic properties. The compounds were screened by carageenin rat paw edema method and showed good anti-inflammatory properties.
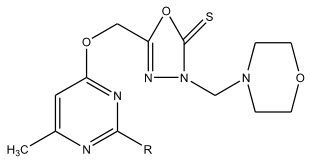
The synthesis of 5-(6-methyl-2-substituted-4-pyrimidinyloxymethyl)-2,3-dihydro-1,3,4-oxadiazol-2-thiones and their morpholinomethyl derivatives were carried out by Virginija Jakubkiene et al 15. The synthesized compounds were evaluated for their anti-inflammatory activity and some of these were more active than acetylsalicylic acid.
Harish kumar et al 16 prepared a series of 1,3,4-oxadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxyaceticacid and evaluated these compounds for their anti-inflammatory, analgesic and lower ulcerogenic potential. The compounds were found to posses strong anti-inflammatory properties.
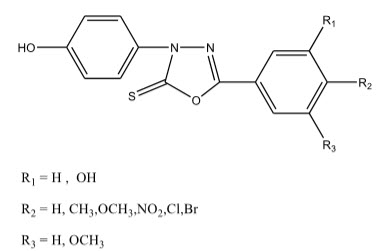
Mohd. Amir et al 17 synthesized a series of 1,3,4-oxadiazole derivatives and 1,2,4-triazine-5-one derivatives. All the compounds were screened for their anti-inflammatory activity by using Carageenin-induced rat paw edema method. Compounds A (1) and A (2) among all the synthesized compounds showed maximum anti-inflammatory activity.
Anticancer activities
Loetchutiant et al 18 synthesized a novel series of 5-(substituted aryl)-3-(4-hydroxyphenyl)-1,3,4-oxadiazole-2-(3H)-thiones by the cyclocondenstaion of 1-(4-hydroxyphenyl)-2-aroylhydrazines with thiophosgene. The synthesized compounds were evaluated for their anti-proliferative activity against micro-organisms. IC50 values ranges from 24 to 94 mM and the compounds were found to have comparable efficacy with that of apigenin and genistein.
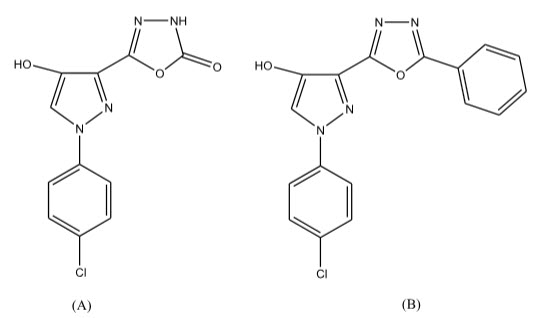
Saddi et al 19 have reported the synthesis of 5-(1-(4-chlorophenyl)-4-hydroxy-1H-pyrazol-3-yl)-1,3,4-oxadiazol-2-one (A) and 5-(1-(4-chlorophenyl)-4-hydroxy-1H_pyrazol-3-yl)2-phenyl-1,3,4-oxadiazole derivatives (B). The compounds were screened for their anti-cancer activity.
Ouyang et al 20 synthesized and evaluated various 1,3,4-oxadiazole derivatives as to their ability to inhibit tubule polymerization and block the mitotic division of tumor cells. Compound A exhibited potent activity. In-vitro studies of compound A indicated that at nano concentrations it interrupted mitotic division in breast carcinoma and squamous cell tumors, which included multi-drug resistant cells.
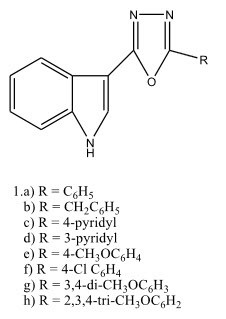
Dalip kumar et al 21 prepared a series of novel 5-(3’-indolyl)-2-(substituted)-1,3,4-oxadiazoles and screened them for their in-vitro anti-cancer activity against human cancer cells from prostate (PC3,DU145 and LnCaP), breast (MCF7 and MDMDA231) and pancreas(PaCa2). The compound 1.a) with two phenyl group exhibited moderate activity against MCF7 and activity against other cell lines. Also N-methylation of indole ring nitrogen dramatically improved the cytotoxic activities.
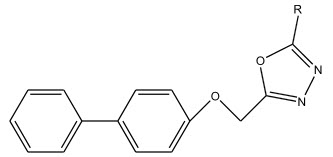
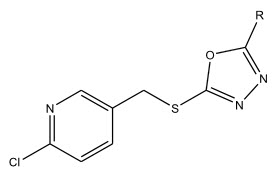
Qing-Zhong Zheng et al 22 synthesized a series of 2-chloropyridine derivatives possessing 1,3,4-oxadiazole moiety. The compounds showed anti-proliferative activity against gastric cancer cell SGC-7901, which was more potent than the positive control.
REFERENCES:
1.Radha R.B and Rao B.U.T. (1990). Synthesis and Biological activities of benzthiazolothiomethyl-oxadiazoles, thiadiazoles and triazoles. Indian Journal of Chemistry, 29, 995 – 998.
2.Duita MM and Kataky (1992). Synthesis and biological activities of Azadianesarylphosphate/ thiophosphate derivatives of 1,3,4-oxadiazoles and thiadiazoles. Indian Journal of Chemical Society, 69, 599 – 601.
3.Kalluraya B , Ramesh C and Shivaram HB (1995). Synthesis and anti-microbial activity of various 5-substituted-1,3,4-oxadiazole-2-hydrazides. Indian Journal of Pharmaceutical Sciences, 5, 37 – 39.
4.Sonar VN , Yadav LDS and Sandhya S. (1995). New Mannich bases from oxadiazolyl indoles and their anti-bacterial activities. Indian Journal of Heterocyclic Chemistry, 4, 203 – 205.
5.Nailesh J., Korgaokar S. and Parekh H. (1996). Synthesis and anti-microbial activity of substituted 1,3,4-oxadiazoles. Indian Journal of Heterocyclic Chemistry, 5, 241 – 242.
6. Jayamma Y., Sarangapani M. and Reddy V.M. (1996). Synthesis and evaluation of biological activity of novel heterocycles. Indian Journal of Heterocyclic Chemistry, 6, 111 – 114.
7. Shah V.R., Vadodaria M. and Parikh A.R. (1997). Synthesis of 1,3,4-oxadiazoles having nicotinamide moiety as potential anti-microbial agents. Indian Journal of Chemistry, 36, 101 – 103.
8.Kapoor RP and Batra H (1997). Synthesis of some oxadiazoles and thiadiazoles of potential biological importance. Indian Journal of Heterocyclic Chemistry, 6, 1 – 4.
9.Chaudary BR , Shinde DB and Shigara MS (1995). Synthesis of some 1,.4-benzothiazinyl thiosemicarbazides, triazoles, oxadiazoles, thiadiazoles and evaluation of their anti-tubercular activities. Indian Journal of Heterocyclic Chemistry, 4 , 187 – 189.
10.Parekh H.H., Preethi K.R., Niraj S.S. and Rajeev D.V. (1999). Synthesis and anti-microbial activity of 2,5-disubstituted-1,3,4-oxadiazoles. Indian Journal of Chemistry, 38, 572 – 574.
11.Kucukguzel S.G., Oric E.E., Rollas S., Sachin F. and Ozbek A. (2002). Synthesis, Characterization and biological activity of novel 4-thiadiazolidinones, 1,3,4-oxadiazoles and some related compounds. European Journal of Medicinal Chemistry, 37, 197 – 206.
12.Shashikant P.R., Rabara P.A., Pattan S. and Jayashri (2009). Synthesis and evaluation of some novel substituted-1,3,4-oxadiazole derivatives for anti-tubercular activity. Indian Journal of Chemistry, 48, 1453 – 1456.
13.Sawhney S.N. , Gupta A. and Sharma P.K. (1991). Synthesis of some new 2-(2-methylthiazol-4-yl)-1,3,4-oxadiazoles and 5-(2-methylthiazol-4-yl)-3-mercapto-1,2,4-triazoles as potent anti-inflammatory agents. Indian Journal of Heterocyclic Chemistry, 1 , 8 – 16.
14.Mullican MD, Wilson MW, Connor DT, Kostlan CR, Schrier DJ and Dyer RD (1993). Design of 5-(3,5-ditertbutyl-4-hydroxyphenyl)-1,3,4-oxadiazoles and -1,3,4- thiadiazoles and -1,2,4-triazoles as orally active , non-ulcerogenic and anti-inflammatory agents. Journal of Medicinal Chemistry,36 , 1090 – 1099.
15.Jakubkiene V., Barbuliene M.M., Kiene G.K., Naite E.U., Gaidelis P. and Vainilavicius P. (2003). Synthesis and anti-inflammatory activity of 5-[6-methyl-2-substituted-4-pyrimidinyloxymethyl)-1,3,4-oxadiazol-2-thiones and their morphilinomethyl derivatives. Il Farmaco, 58, 323 – 328.
16.Kumar H., Javed S.A., Khan S.A. and Amir M. (2008). 1,3,4-oxadiazole/ thiadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxy acetic acid : Synthesis and preliminary evaluation of biological properties. European Journal of Medicinal Chemistry, 43, 2688 – 2698.
17.Amir M., Kumar H., Javed S.A. and Khan S.A. (2008). Synthesis, characterization and screening of novel 1,3,4-oxadiazole derivatives for analgesic, anti-inflammatory and anti-microbial activity. European Journal of Medicinal Chemistry, 43, 2688 – 2698.
18.Loetchutiant C., Chau F. and Mankhetkom S. (2003). Synthesis and evaluation of 5-aryl-3-(4-hydroxyphenyl)-1,3,4-oxadiazol-2-(3H)-thiones as P Glycoprotien inhibitors. Chem. Pharma Bull, 51, 728 – 730.
19.Saddi M.S.A., Rustam S.A.F. and Allah H.M.F. (2005). In-vitro anti-tumour screening of some polysubstituted pyrazole analogues. Saudi Pharma Journal, 13, 89 – 96.
20.Ouyang X., Patnitsk E.L., Pattaropong V., Chen X., Kiselyov A.S., Velangar A., Kaurkami J., Labelle M. and Smith L. (2006). Oxadiazole derivatives as a novel class of anti-mitotic agents : Synthesis, inhibition of tubulin polymerization and activity in tumor cell lines. Biorg. Med. Chem. Lett., 16, 1191 – 1196.
21.Kumar D., Sundaree S., Johnson E.O. and Shah K. (2009). An efficient synthesis and biological study of novel indolyl-1,3,4-oxadiazoles as potent anti-cancer agents. Biorganic and Medicinal Chemistry Letters, 19, 4492 – 4494.
22.Zheng Q.Z., Zhang X.M., Cheng Y.X.K., Jiao Q.L. and Zhu H.L. (2010). Synthesis, biological evaluation and docking studies of 2-chloropyridine derivatives possessing 1,3,4-oxadiazole moiety as potential anti-tumour agents. Biorganic and Medicinal Chemistry, 18, 7836 – 7841.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









