{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Ankit J. Joshi
Department of Pharmaceutics & Pharmaceutical Technology,
S. K. Patel College of Pharmaceutical Education and Research,
Ganpat University, Ganpat vidyanagar, Kherva, Mehsana-Gozaria Highway, Gujarat.
Joshiankit2824@gmail.com
ABSTRACT
The preservation of cells, tissues and organs by cryopreservation is promising technology now days and Low temperature technology has progressed in the field of tissue engineering, food preservation, fertility preservation, making disease resistant breeds since the early years to occupy a central role in this technology. Cryopreservation is important technology in every field like in organ cryopreservation, food cryopreservation, human cryopreservation, seeds cryopreservation, protein cryopreservation and pharmaceuticals. Cryobiologists will be required to collaborate with new physical and molecular sciences to meet this challenge. How cryoprotectants work is a mystery to most people. In fact, how they work was even a mystery to science until just a few decades ago. This article will explain in basic terms how cryoprotectants protect cells from damage caused by ice crystals, and with some of the advances.
INTRODUCTION
A cryoprotectant is a substance used to protect biological tissue from freezing damage (i.e. that due to ice formation). Cryopreservation is a process where cells or tissues are preserved by cooling to low sub-zero temperatures, such as 77 K or -196°C (the boiling point of liquid nitrogen). Cryopreservation is the use of very low temperatures to preserve structurally intact living cells and tissues. Unprotected freezing is normally lethal. Theories of freezing injury have envisaged either that ice crystals pierce or tease apart the cells, destroying them by direct mechanical action, or that damage is from secondary effects via changes in the composition of the liquid phase. Cryoprotectants, simply by increasing the total concentration of all solutes in the system, reduce the amount of ice formed at any given temperature; but to be biologically acceptable they must be able to penetrate into the cells and have low toxicity.At these low temperatures the biochemical reactions is effectively stopped that would otherwise leads to damage to tissue or cell death. However, when cryoprotectant solutions are not used, the cells being preserved are damaged due to freezing or thawing during the approach to low temperatures or warming to room temperature [1, 2]
Types of cryopreservation[1-4]
(1) Isochoric cryopreservation
Isochoric (constant volume) cryopreservation is referred for biological material at low temperature. In isochoric cryopreservation, the solution concentration is lower than the isobaric cryopreservation at all temperature. Isochoric cryopreservation is very simple; freezing is done in a constant volume chamber.
Since the reduction of metabolic rates is a strong function of temperature, so result of decrease metabolic activity for every 10 degree Celsius decrease in temperature it is most desirable to preserve biological materials close to absolute zero. In it the system naturally adjusts at the minimal pressure for the particular cryopreservation temperature. Using a simple isochoric cryopreservation device, we confirm the theoretical thermodynamic predictions.
(2) Isobaric cryopreservation
Isobaric (constant pressure) process, occur at a pressure of 1 atm. which is constant atmospheric temperature and also implement on Earth and reduce chemical damage at high subzero or negative temperatures by adding compounds that depress the freezing point of the solution, can penetrate the cell membrane.
(3) Hyperbaric cryopreservation
It also reduce the ionic concentration at subzero Celsius temperatures. Like isochoric cryopreservation, it also leads to elevated pressures, thus elevated pressures, followed by rapid freezing lead to reduction of ice crystal and preservation of the biological material structure. Several tissues are preserved by using hyperbaric pressure at low subzero temperature e.g., kidney, liver, cornea, blood cells or cell preservation. Survival of cells is directly proportional to magnitude of the pressure. As increases the pressure survival of cells also increases e.g. Kidney (1000 atm.), cells (200 atm.), liver (70MPa) pressure limit are adjusted.
Damage or risk during cryopreservation
The damage of cells during cryopreservation mainly occurs during the freezing stage include: solution effects, extracellular ice formation, dehydration and intracellular ice formation. Many of these effects can be reduced by Cryoprotectants. (Show figure 1)
Solution effects
The damage caused by chemical and osmotic effects of concentrated solutes in the residual unfrozen water between ice crystals. This is so-called “solution effects” injury. During freezing, ice crystals form internally that excludes the solutes which remains in external liquid as externally concentration of solute is high that causes damage.
Extracellular ice formation
At slow cooling rate, water migrates out of cells and ice crystal forms extracellular. This will cause mechanical damage to the cell.
Dehydration
The migration of water causing extracellular ice formation which will cause cellular dehydration.
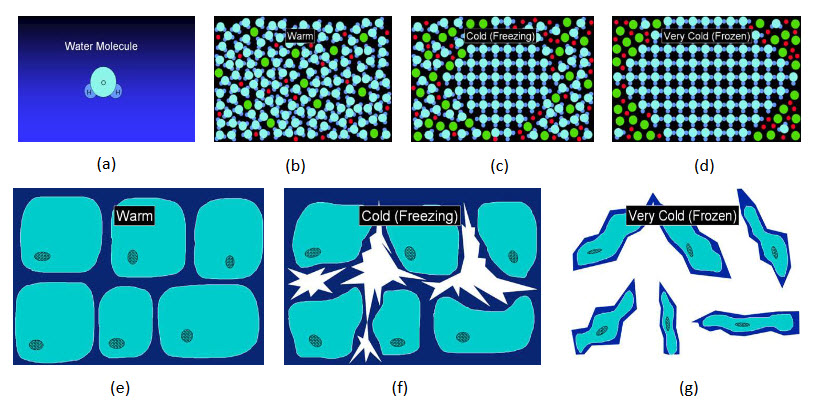
Figure 1: Graphical presentation of damage
(a) Living tissue contains much of water. (b) Water is component of a cellular solution in living tissue. (c) When tissue is cooled below freezing temperature, water molecules gather together and form growing ice crystals. (d) Growing ice expel other molecules from the ice lattice to form a harmful concentrated solution. (e) On a cellular scale, ice forms first outsidecells. (f) Growing ice causes cells to dehydrate and shrink. (g) Finally cells are left damaged and squashed between ice crystals. The damage is mostly mechanical.
How Cryoprotectants Protect Cells:
1. Adding cryoprotectants to cellular solution can prevent crystallization of water to ice.
2. Instead of freezing, molecules just move slower and slower as they are cooled.
3. Finally, at very low temperature, molecules become locked in place and an amorphous solid is formed. Water that becomes solid without freezing is said to be "vitrified".
4. There is no damage to cells during cooling because no ice is formed.
Mechanism of cryoinjury[5-7]
Mazur’s two-factor hypothesis revealed two mechanisms of cell injury during freezing and thawing;
One occurring at cooling rates where the cell remains close to osmotic equilibrium and the other at rates in which there is super cooled water within the cell.
Graded Freezing technique is used to separate above two type of injury. The graded freezing technique require following steps:
1. Sample is slowly cooled
2. Two sample is removed at different temperature
3 .one samples is thawed
4. Second sample is dip in liquid nitrogen
Main methods to prevent risks [10]
1) Controlled rate and slow freezing
This method involves a brief pre-equilibration of cells in cryoprotectant solutions followed by slow, gradual, controlled cooling at rates optimized for the type of cells being cryopreserved. The whole process is carried out with the use of special programmable cell freezing equipment and requires 3-6 hours to complete. Cryoprotectants are used to protect the cells from damage due to intracellular ice crystal formation. The temperature of the cells is lowered to a super cooled state and ice crystal growth is initiated within the extra-cellular solution by a process called seeding.
As the size of the ice crystals increases, water in the solution is converted from liquid state to solid. This increases the concentration of solute in the extracellular medium which draws water out of the cell. As a result the cell dehydrates and this increased intracellular solute concentration further lowers the freezing point of the cell to approximately -350 C. The cell is almost devoid of any water at this point and therefore ice crystal formation is negligible when the cell ultimately freezes at this temperature. The rate at which water leaves the cell depends on the rate of cooling.
When the cells are cooled at rapid rate, water present inside the cell will not be able to move out fast enough leading to the formation intracellular ice crystals which are lethal to the cell. If the cells are cooled too slowly, then there will be severe volume shrinkage leading to high intracellular solute concentration, which have deleterious effects on the lipid-protein complexes of cell membranes. In addition the cells that are cooled slowly are potentially affected by chilling injury.
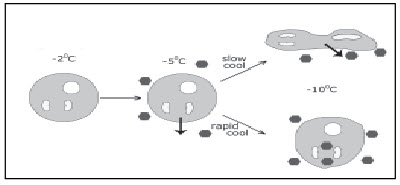
Figure 2: Influence of rate of cooling on intracellular ice formation.
Hence the rate of cooling and cryoprotectant concentration employed in the protocol should be optimized to avoid the intracellular ice crystallization and high solute concentration, the two main events involved in cellular injury during cryopreservation.
The success of slow cooling depends on achieving this optimal balance between the rate at which water can leave the cell and the rate at which it is cooled before it is converted into ice. Therefore, to achieve this balance, the time to complete slow cooling procedures for human oocytes and embryos needs a minimum of 90 min. Besides the longer time needed, the slow cooling protocol requires expensive programmable freezing equipment. Apart from these limitations slow cooling protocols are not satisfactory for various types of cells viz, pig embryos, in vitro derived bovine embryos, human MII oocytes and blastocysts which are sensitive to chilling injury.
2) Flash-freezing process (vitrification)
This method of cryopreservation was developed to overcome the shortcomings of slow freezing protocol. It is the solidification of a solution at low temperature, not by ice crystallization, but by extreme elevation in its viscosity using high cooling rates of 15,000 to 30,0000 C/min. The cooling of cells at this ultra high rate of freezing creates a glass like state without intracellular ice formation. Thus, the term vitrification, which means ‘turned into glass’ was first proposed by Luyet (1937). During vitrification the viscosity of the cytosol becomes greater and greater until the molecules become immobilized and it is no longer a liquid, but rather has the properties of a solid’. [11]
Vitrification involves exposure of the cell to high concentration of cryoprotectants for a brief period at room temperature followed by rapid cooling in liquid nitrogen. The cells are initially pre-equilibrated in a cryoprotectant solution of lower strength (usually 10 %) resulting in dehydration of the cell and its permeation with cryoprotectant. This is followed by a very short incubation (<30 seconds) in higher concentration of cryoprotectant solution (40%) followed by rapid plunging into liquid nitrogen. The high osmolarity of the cryoprotectants results in complete dehydration of the cell. Since the cells are almost devoid of any water by the time they are immersed in liquid nitrogen, the remaining intracellular water, if any, does not form ice crystals. During warming the entire process of vitrification of the cell is reversed. Cells are exposed in a step wise manner to hypotonic solutions of decreasing strengths of sucrose to remove the cryoprotectant and gradually rehydrate.
Properties of Cryoprotectants:
Not all chemicals that dissolve in water are cryoprotectants. In addition to being water soluble, good cryoprotectants are effective at depressing the melting point of water, do not precipitate or form eutectics or hydrates, and are relatively non-toxic to cells at high concentration.
All cryoprotectants form hydrogen bonds with water. Since the discovery of glycerol as the first cryoprotectant more than 50 years ago the best and most commonly used cryoprotectants are classes of cryoprotectants called penetrating cryoprotectants.
Penetrating cryoprotectants are small molecules that easily penetrate cell membranes. The molecular mass of penetrating cryoprotectants is typically less than 100 daltons. By entering and remaining inside cells, penetrating cryoprotectants prevent excessive dehydration of cells during the freezing process.
Component of cryopreservation solution [12-13]
A cryopreservation solution, which may be either a freezing solution or vitrification solution, consists of:
Carrier Solution: Carrier solution consists of solution ingredients that are not explicit cryoprotectants. The role of the carrier solution is to provide basic support for cells at temperatures near freezing. It contains salts, osmotic agents, pH buffers, and sometimes nutritive ingredients or apoptosis inhibitors. The ingredients are usually present at near isotonic concentration (300 milliosmoles) so that cells neither shrink nor swell when held in carrier solution. Carrier solution is sometimes called “base perfusate.”
The carrier solution typically used with M22 cryoprotectant solution is called LM5. Different concentrations of cryoprotectant may be required at various stages of cryoprotectant introduction and removal, but the concentration of carrier solution ingredients always remains constant. This constant-composition requirement can be regarded as the definition of a carrier solution. As a practical matter, this means that cryopreservation solutions must be made by means other than adding cryoprotectants to a pre-made carrier solution because native addition would dilute the carrier ingredients.
Ice Blockers (Optional Ingredient): Ice blockers are compounds that directly block ice growth by selective binding with ice or binding to contaminants that trigger ice formation (ice nucleators). Conventional cryoprotectants act by interacting with water. Ice blockers compliment conventional cryoprotectants by interacting with ice or surfaces that resemble ice. Ice blockers are like drugs in that only a small amount is required to find and bind their target. Low molecular weight polyvinyl alcohol and polyglycerol, called X-1000 and Z-1000, and biological antifreeze proteins are examples of ice blockers. Ice blockers are only used in vitrification solutions, not freezing solutions
Classification
These are usually separated into two broad classes based on their ability to diffuse across cell membranes. Penetrating cryoprotectant are able to move across cell membranes whereas non-penetrating agents cannot.
Penetrating Cryoprotectant: Penetrating cryoprotectants are small molecules able to cross cell membranes. The role of penetrating cryoprotectants is to reduce ice growth and reduce cell dehydration during freezing. In vitrification, the role of penetrating cryoprotectants is to completely prevent ice formation. Penetrating cryoprotectants are the majority ingredients of vitrification solutions. (Ex.: DMSO)
Non-Penetrating Cryoprotectants: Non-penetrating cryoprotectants are large molecules, usually polymers, added to cryoprotectant solutions. They inhibit ice growth by the same mechanisms as penetrating cryoprotectants, but do not enter cells. Polyethylene glycol (PEG) and polyvinylpyrrolidone (PVP) are examples. Non-penetrating cryoprotectants are usually less toxic than penetrating cryoprotectants at the same concentration. They reduce the amount of penetrating cryoprotectants needed by mimicking outside the cell the cryoprotective effects of proteins inside the cell. It has also been recently discovered that using non-penetrating cryoprotectants to increase the tonicity (osmotically active concentration) of vitrification solutions can prevent a type of injury called chilling injury. (Ex.: Starch, trehalose)
Other examples based on carbohydrates and polyols:
|
Cryoprotectant |
Examples |
|
Monosaccharide |
Fructose, Dextrose |
|
Disaccharide |
Sucrose, lactose, inuline |
|
Oligosaccharide |
Cellobiose,Trehalose |
|
Polysaccharide |
Starch,Chitosan |
|
Amino acid |
Phenylalanin,Tyrosin |
|
Polyols |
Glycerol |
|
Other |
Dimethyl sulfoxide (DMSO) and its derivative |
Application & Recent Advances [14-18]
1. Advances in organ freezing
The main new initiatives in organ freezing have been efforts to successfully freeze whole ovaries to preserve fertility against the risks posed by chemotherapy in youth or by aging. Seven rat ovaries, still attached to their fallopian tubes and the upper segment of the uterus, were perfused with 0.1M fructose and dimethyl sulfoxide (DMSO) to attain a final DMSO concentration of 1.5M (just a bit over 10% by volume) over 30 minutes, frozen slowly, and stored in liquid nitrogen overnight before thawing, gradual cryoprotectant washout, and vascular transplantation. Three of seven thawed grafts had no follicles, and the 4 grafts that did have follicles had reduced numbers of follicles and recipients had reduced estrogen levels, elevated FSH levels, and reduced uterine weights, but the 4 surviving ovaries were able to ovulate and one animal was able to get pregnant and have two pups (reduced litter size).
This was good enough for Nature, and, interestingly, the microscopic structure of the fallopian tubes and uterine portion of the transplants was normal. Clearly, freezing was detrimental, and the authors suggested that vitrification might work better, but just as clearly, at least portions of these ovaries and all of the associated structures survived freezing and thawing.
2. Depressed Metabolism
Cryopreservation may find the increasing use of the term suspended animation for therapeutic interventions like whole body (ultra) profound asanguineous hypothermia and normothermic hypometabolism indicative of a lack of precision. But the increased support for and research in these areas in mainstream biomedical science and the media may produce a more favorable reception of research aimed at reversible human cryopreservation and real suspended animation as a result. Another advantage of increased research efforts in these areas is that cryonics providers can benefit from these findings to enhance their own capabilities and initiate informed research into improved organ preservation solutions and “hibernation mimetics.”
3. Nosy Neuroprotection: Intranasal Delivery of Neuroprotective Agents to the Brain
In cryonics, certain neuroprotective agents are administered to patients in an attempt to prevent (or at least slow down) ischemic damage to the brain after cardiac arrest and during the low flow reperfusion provided by cardiopulmonary support. Cooling is, of course, our primary line of defense against such damage because of its striking effectiveness in reducing metabolic demand. However, rapid field cooling still presents considerable logistical and clinical challenges and preferential brain cooling is (at least until the patient arrives in the operating room) yet to be accomplished.
Therefore, quick and direct protection of the brain is especially important in the moments following pronouncement and during the initial stages of cooling, while the patient is still relatively warm. Currently, all medications delivered to cryonics patients are introduced to the systemic circulation either via the intravenous (IV) or intraosseous (IO) routes, requiring skilled personnel who have been trained in IV and/or IO technique. Due to the blood-brain barrier and first-pass metabolism,relatively large volumes of neuroprotective agents must be given in order for an effective dose to enter the brain via the systemic circulation. In fact, some of the most promising neuroprotective drugs are currently not available at all for treatment of cryonics patients because of poor BBB permeability.
4. Cryopreservation of Human Embryonic Stem Cell Lines
Two different approaches have been adopted for the cryopreservation of human embryonic stem cells (hESCs): vitrification and conventional slow cooling/rapid warming. The vitrification method described here is designed for hESCs that grow as discrete colonies on a feeder cell monolayer, and are subcultured by manual subdivision of the colonies into multicellular clumps. hESCs that are subcultured by enzymatic dissociation can more conveniently be cryopreserved by conventional slow cooling/rapid warming methods.
Although both methods are suitable for use in a research context, neither is suitable for cryopreservation of embryonic stem cells destined for clinical diagnostic or therapeutic uses without modification.
5. Cryopreservation of Shoot Tips and Meristems
Shoot-tip meristem cryopreservation methodologies are reported for the complementary cryoprotective strategies of vitrification and equilibrium freezing using traditional controlled-rate freezing and chemical additive cryoprotection. Pregrowth, pretreatment, and cold acclimation approaches for the improvement of tolerance to liquid nitrogen are also presented. The article concludes by reporting an analytical protocol that profiles volatile hydrocarbon stress markers (for ethylene, hydroxyl radicals, and lipid peroxidation products) during cryopreservation. This method uses noninvasive headspace sampling and gas chromatography, and it is widely applicable across cryogenic systems.
6. Cryopreservation of Red Blood Cells and Platelets
Blood cells can be regarded as a classical field of application of low-temperature biology. Cryopreservation methods have been developed for different categories of blood cells namely red blood cells (RBCs) (erythrocytes), platelets (thrombocytes), mononuclear cells (i.e., lymphocytes, monocytes), and hematopoietic progenitor cells.
7. Cryopreservation of Mammalian Oocytes
Two methods for the cryopreservation of mammalian oocytes are described. One method uses a relatively low concentration of the cryoprotectant propanediol plus sucrose and requires controlled-rate cooling equipment to achieve a slow cooling rate. Such a method has produced live births from cryopreserved human oocytes.
The second method described employs a high concentration of the cryoprotectant dimethyl sulfoxide plus a low concentration of polyethylene glycol. This method involves cooling by plunging standard straws into liquid nitrogen vapor, hence avoiding the need for specialized equipment,but requires technical ability to manipulate the oocytes quickly in the highly concentrated cryoprotectant solutions. Murine oocytes vitrified, using this technique, have resulted in live births.
CONCLUSION
Applied low temperature technology has progressed significantly since the early years to occupy a central role in many modern scientific endeavors. While it much has been learnt about the role of CPA and their mode of action, there still remain significant gaps in our understanding about their molecular interactions with cell components and potential toxicities. Cryobiology and cryonics are at the interface between physics, chemistry, and biology the knowledge of physical-chemistry laws is very important. While at present there is much uncertainty about whether cryonics is justifiable; a personal choice is still the governing force in the matter. Cryobiologists will be required to collaborate with new physical and molecular sciences to meet this challenge. However, if improvements are made to the current cryopreservation techniques, it will possibly be used to preserve smaller entities without any damages. Being able to maintain vital organs (such as liver, lungs, heart, kidneys, skin, and bone marrow) in good conditions for extended periods would be remarkable advances in medicine, allowing the creation of organ banks, and therefore reducing the waiting time for organ transplantation.
REFERENCE ID: PHARMATUTOR-ART-2381
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 1 Received On: 12/08/2015; Accepted On: 27/08/2015; Published On: 01/01/2016How to cite this article: Joshi AJ; A Review and Application of Cryoprotectant: The Science of Cryonics; PharmaTutor; 2016; 4(1); 12-18 |
REFERENCES
1. Karlsson M.O., Toner M; Introduction and application of Cryopreservation tech. in tissue engineering; Principle of tissue engg; 2(1); 293-304
2. Preciado J.A.,Rubinsky B.; Isochoric preservation; A novel characterization method; Cryobiology; 2010; 60(1); 23-29
3. Rubinsky B.,Szobota S.A.; The Thermodynamic principle of isochoric cryopreservation; Cryobiology; 2005; 50(2); 121-138
4. Rudolph A.S., Crowe J.H.; Membrane stabilizing during freezing; Cryobiology; 1985; 22; 367-377
5. Osetsky A.l.; Thermodynamic aspects of cluster crystallization in cryoprotective solution; cryo letters; 2011; 32(3); 216-24
6. Fahy M; Cryoprotectant toxicity and cryoprotectant toxicity reduction; Cryobiology; 1990; 27(3); 247-268.
7. Leibo S.P.; The principle variable of cryopreservation; Fertile Steril.; 2011; 96(2); 269-276
8. Weng L., Li W.,Chan C.,; Kinetics of Coupling Water and Cryoprotectant transport across cell membrane and Application to cryopreservation; Phys. chem. B; 2011; 115(49); 14721-14731
9. Son Young Weon; comparison of slow freezing method with vitrification; Expert rev. Med. Devices; 2009; 6(1); 1-7
10. Fahy G.M.; Vitrification: a new approach to organ cryopreservation. In: Transplantation: approaches to graft rejection; Alan R Liss; 1986; 305-335
11. Wowk B., Leitl E., Rasch C.M., Mesbah-Karimi N., Harris S.B., Fahy G.M., Vitrification enhancement by synthetic ice blocking agents; Cryobiology; 2000; 40(3); 228-236.
12. Wowk B., Fahy G.M.; Inhibition of bacterial ice nucleation by polyglycerol polymers; Cryobiology; 2002; 44(1); 14-23
13. Gndolfi F, Efficiency of equilibrium cooling and rapid freezing procedure for cryopreservation of rat ovarian tissue Fertility and sterility; 2006;81(1),1150-1156
14. Szczygiel M.A.; Intracytoplasmic sperm injection is more efficient than in vitro fertilization for generating mouse embryo from cryopreserved spermatozoa; Biol. Reprod; 2002; 67(4); 1278-1284
15. Esteves S.C.; Suitability of the hypo-osmotic swelling test for assessing the viability of cryopreserved sperm, fertile. steril; 1996; 66(5); 798-804.
16. Smith G.D.,Motta E.E., Serafini P.; Theoretical and experimental basis of oocytes vitrification; Reprod biomed online; 2011; 23(3); 298-306.
17. Benson EE., Withers LA.; Gas chromatographic analysis of volatile hydrocarbon production by cryopreserved plant tissue cultures—a nondestructive method for assessing stability; CryoLetters; 1987; 8(1); 35–46.
18. Harding K., Benson E. E., Smith H.; The effects of tissue culture duration on post-freeze survival of shoot-tips of Solanum tuberosum.; CryoLetters; 1991; 12(1); 17–22.
19. Acker J.P., Chen T., Fowler A., Toner M., Fuller B.J., Lane N.J., Benson E. E.; In Life in the Frozen State; CRC Press, Boca Raton, FL; 2004; 563-580.
20. Upper C.D., Vali G., Lee R.E., Warren G.L., Gustav L.V.; In Biological Ice Nucleation and Its Application, Am Phytopathol Soc; St Paul MN; 1995; 1-28.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









