 About Authors:
About Authors:
Apeksha Gupta
Maharshi Dayanand University,
Rohtak, India.
apekshagupta87@gmail.com
ABSTRACT
The word “Dossier” has its English meaning as - a collection or file of documents on the same subject, especially a file containing detailed information about a person or a topic. Any preparation for human use that is intended to modify or explore physiological systems or pathological states for the benefit of the recipient is called as “pharmaceutical product for human use”. Process of reviewing and assessing the dossier of a pharmaceutical product containing its detailed data (administrative, chemistry, pre-clinical and clinical) and the permission granted by the Regulatory Agencies of a country with a view to support its marketing / approval in a country is called as the “Marketing Approval or the “Registration” “Marketing Authorization” or the “Product Licensing”.
“Registration Dossier” of the pharmaceutical product is a document that contains all the technical data (administrative, quality, nonclinical and clinical) of a pharmaceutical product to be approved / registered / marketed in a country. It is more commonly called as the New Drug Application (NDA) in the USA or Marketing Authorization Application (MAA) in the European Union (EU) and other countries, or simply Registration Dossier. Basically, this consists of data proving that the drug has quality, efficacy and safety properties suitable for the intended use, additional administrative documents, samples of finished product or related substances and reagents necessary to perform analyzes of finished product. Therefore, they are the vehicle in a country through which drug sponsors formally propose that the Regulatory Agencies approve a new pharmaceutical for sale and marketing.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1485
The goals of the dossiers are to provide enough information to permit Regulatory Agencies’ reviewers to establish the following:
• Is the drug safe and effective in its proposed use(s) when used as directed, and do the benefits of the drug outweigh the risks?
• Is the drug’s proposed labelling (package insert) appropriate, and what it contain?
• Are the methods used in manufacturing (Good Manufacturing Practice, GMP) the drug and the controls used to maintain the drug’s quality adequate to preserve the drug’s identity, strength, quality, and purity?
Every country has its own regulatory authority, which is responsible to enforce the rules and regulations and issue the guidelines to regulate the marketing of the drugs. The single regulatory approach for marketing authorization application (MAA) of a new drug product applicable to various countries (on the basis of single dossier) is utmost difficult. Therefore, the knowledge of exact and detailed regulatory requirements for MAA of each country should be known to establish a suitable regulatory strategy.
INTRODUCTION
A regulatory process, by which a person/organization/sponsor/innovator gets authorization to launch a drug in the market, is known as drug approval process. In general, a drug approval process comprises of various stages: application to conduct clinical trials, conducting clinical trials, filing of Registration Dossier/ New Drug Application (NDA) and post-marketing studies. Every country has its own regulatory authority, which is responsible to enforce the rules and regulations and issue the guidelines to regulate the marketing of the drugs. The single regulatory approach for marketing authorization of a new drug product applicable to various countries (on the basis of single dossier) is utmost difficult. Therefore, the knowledge of exact and detailed regulatory requirements for Registration Dossier of each country should be known to establish a suitable regulatory strategy.
Drug registration implements one of the legal requirements for marketing of drugs in a country. Drug registration guidelines provide guidance to applicants who may wish to market their pharmaceutical products in the market. They intend to assist applicants in the preparation of acceptable application documents. It is therefore essential that every person who intends to market a medicinal product in country reads the whole of these guidelines carefully and follows strictly the instructions prescribed herein. Submission of applications, which do not comply with the prescribed requirements, may result in delays, queries or rejection of registration.
The content and format of the dossier must follow rules as defined by the Competent Authorities.
REGULATORY DOSSIER SUBMISSION IN ICH COUNTRIES
The complete name of ICH is the "International Conference on harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use". ICH is a joint initiative involving both regulators and research-based industry representatives of the European Union, Japan and the USA in scientific and technical discussions of the testing procedures required to assess and ensure the safety, quality and efficacy of medicines. The goal of ICH is to promote international harmonization by bringing together representatives from the three ICH regions (EU, Japan and USA) to discuss and establish common guidelines.
For example, since year 2003, the authorities in the United States, the European Union (EU) and Japan ask for the Common Technical Document (CTD) format set out by the 2003 International Conference on Harmonization (ICH) which was agreed by the Regulatory Agencies of Europe, Japan and the US and the Research-based Industryand more recently, its electronic version - the electronic Common Technical Document(eCTD). CTD provides a common format for the submission of information to the Regulatory Agencies for the registration of the pharmaceutical product.
The CTD is organized into five modules as shown in Figure I. Module 1 is region specific and Modules 2, 3, 4 and 5 are intended to be common for all regions. The agreement to assemble all the Quality, Safety and Efficacy information in the CTD format has revolutionized the regulatory review processes, led to harmonized electronic submission that, in turn, enabled implementation of good review practices.
Figure I: CTD Triangle
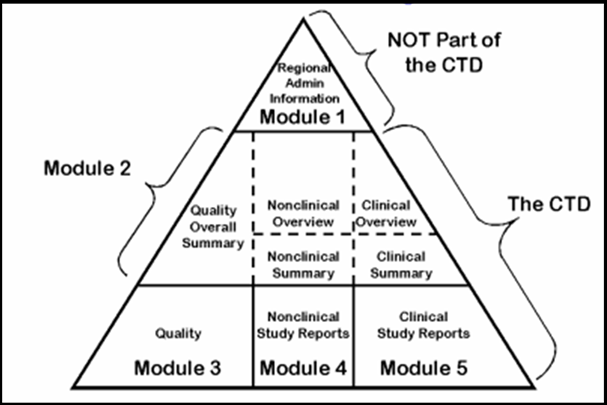
Module 1: Administrative Information and Prescribing Information
1.1 Table of Contents of the Submission Including Module 1.
1.2 Documents Specific to Each Region e.g. the application forms, labeling etc
Module 2: CTD Summaries
This module should begin with a general introduction to the pharmaceutical, including its pharmacologic class, mode of action, and proposed clinical use, not exceeding one page.
Module 2 should contain 7 sections in the following order:
· CTD table of contents
· CTD introduction
· Quality overall summary
· Nonclinical overview
· Clinical overview
· Nonclinical written and tabulated summaries
· Clinical summary
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Module 3: Quality
Information on Quality should be presented in the structured format as described below:
3.1 Table of Contents of Module 3
3.2 Body of Data
3.2. S Drug substance(s)
3.2. S.1 General Information
3.2. S.2 Manufacture of Drug Substance (name, manufacturer)
3.2. S.3 Characterization of Drug Substance (name, manufacturer)
3.2. S.4 Quality Control of Drug Substance (name, manufacturer)
3.2. S.5 Reference Standards or Materials (name, manufacturer)
3.2. S.6 Container Closure System (name, manufacturer)
3.2. S.7 Stability of Drug Substance (name, manufacturer)
3.2. P Drug product (name, dosage form)
3.2. P.1 Description and Composition of the Drug Product (name, dosage form)
3.2. P.2 Pharmaceutical Development (name, dosage form)
3.2. P.3 Manufacture of drug product (name, dosage form)
3.2. P.4 Controls of Excipients (name, dosage form)
3.2. P.5 Control of Drug Product (name, dosage form)
3.2. P.6 Reference Standards or Materials (name, dosage form)
3.2. P.7 Container Closure System (name, dosage form)
3.2. P.8 Stability (name, dosage form)
3.2. A Appendices
3.2. A.1 Facilities and Equipment (name, manufacturer)
3.2. A.2 Adventitious Agents for Safety Evaluation (name, dosage form, manufacturer)
3.2. A.3 Excipients
3.3 Literature References
Module 4 : Nonclinical Study Reports
The nonclinical study reports should be presented in the order described below:
4.1 Table of contents of module 4
4.2 Study reports
4.2.1 Pharmacology
4.2.2 Pharmacokinetics
4.2.3 Toxicology
4.3 Literature references
Module 5 : Clinical Study Reports
The human study reports and related information should be presented in the order described below:
5.1 Table of contents of module 5
5.2 Tabular listing of all clinical studies
5.3 Clinical study report
5.4 Literature References
REGULATORY DOSSIER SUBMISSION IN ASEAN
Association of South –East Asian Nations (ASEAN) follows ASEAN – CTD. ASEAN is a geo-political and economic organization of ten countries located in Southeast Asia as shown in Figure II, which was formed on August 8, 1967 by Indonesia, Malaysia, Singapore and Thailand. Since then the membership has extended to Brunei, Myanmar, Philippines, Cambodia, Laos PDR and Vietnam. Even though some of the individual ASEAN countries have their own drug registration formats, all ASEAN countries accept the ACTD.
Figure II: ASEAN Member States
ASEAN Common Technical Dossier (ACTD) provides a common format for the preparation of well-structured Common Technical Dossier applications that will be submitted to ASEAN regulatory authorities for the registration of pharmaceuticals for human use. CTD format significantly reduce the time and resources needed to compile applications for registration and in the future, will ease the preparation of electronic documental submissions. Regulatory reviews and communication with the applicant is facilitated by a standard document of common elements.
The ASEAN Common Technical Document is organized into four parts. The ACTD consists of Parts I to IV whereas ICH – CTD has 5 Modules. The administrative data of Part I is part of ACTD whereas Module 1 of ICH – CTD is purely country specific. The summaries of the quality (Part II), nonclinical (Part III) and clinical (Part IV) are located at the beginning of each part of the ACTD. The ICH – CTD dedicates these summaries in a separate Module 2. As the ACTD does not have such summary part, it consists of only 4 Parts and not 5.
The ASEAN Common Technical Document is organized into four parts.
Part I. Table of Contents, Administrative Data and Product Information
Part I contains initially the overall Table of Contents of the whole ACTD to provide basically the information that could be looked through respectively. Secondly, the next content is the Administrative Data where required specific documentation in details is put together such as application forms, label, and package insert etc. The last section of this part is Product Information where necessary information includes prescribed information, mode of action, side effects etc.
A. Introduction
B. Table of Contents
C. Administrative Data and Product Information
1. Application Form
2. Letter of Authorization
3. Certifications
4. Labelling
5. Product Information
Part II. Quality Document
Part II should provide the Overall Summary followed by the Study Reports. The quality control document should be described in details as much as possible.
Section A: Quality Overall Summary (QOS): Table I gives a view of the Quality Overall Summary of Part II of ACTD.
Table I: Quality Overall Summary of Part II of ACTD
|
S DRUG SUBSTANCE |
P DRUG PRODUCT |
|
S1 General Information |
P1 Description and Composition |
|
S2 Manufacture |
P2 Pharmaceutical Development |
|
S3 Characterization |
P3 Manufacture |
|
S4 Control of Drug Substance |
P4 Control of excipients |
|
S5 Reference Standards or Materials |
P5 Control of Finished Product |
|
S6 Container Closure |
P6 Reference Standards or Materials |
|
S7 Stability |
P7 Container Closure |
|
|
P8 Stability |
|
|
P9 Product Interchangeability Equivalence evidence |
Section B: Table of Contents
Section C: Body of Data
Part III. Nonclinical Document
Part III should provide the Nonclinical Overview, followed by the Nonclinical Written Summaries and the Nonclinical Tabulated Summaries. The document of this part is not required for Generic Products, Minor Variation Products and some Major Variation Products. For ASEAN member countries, the Study Reports of this part may not be required for NCE, Biotechnological Products and other Major Variation Products if the Original Products are already registered and approved for market authorization in Reference Countries.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Section A: Table of Contents
Section B: Nonclinical overview
- Overview of the Nonclinical Testing Strategy
- Pharmacology
- Pharmacokinetics
- Toxicology
- Integrated Overview and Conclusions
- List of Literature Citations
Section C: Nonclinical written and tabulated summaries
1. Introduction
2. Content of nonclinical written and tabulated summaries
- Pharmacology
- Pharmacokinetics
- Toxicology
Section D: Nonclinical study reports
Part IV Clinical Document
Part IV should provide the Clinical Overview and the Clinical Summary. The document of this part is not required for Generic Products, Minor Variation Products and some Major Variation Products. For ASEAN member countries, the Study Reports of this part may not be required for NCE, Biotechnological Products and other Major Variation Products if the Original Products are already registered and approved for market authorization in Reference Countries.
Section A. Table of contents
Section B. Clinical overview
- Product Development Rationale
- Overview of Biopharmaceutics
- Overview of Clinical Pharmacology
- Overview of Efficacy
- Overview of Safety
- Benefits and Risks Conclusions
Section C: Clinical summary
- Summary of biopharmaceutic studies and associated analytical methods
- Summary of clinical pharmacology studies
- Summary of clinical efficacy
- Summary of clinical safety
- Synopsis of individual studies
Section D: Tabular listing of all clinical studies
Section E: Clinical study reports
OVERVIEW OF ICH – CTD AND ACTD
In the present section an attempt has been made to compare the drug regulatory approval procedure and requirements for the registration of pharmaceuticals for human use ICH countries and ASEAN. The main points of divergence are in the content and format of the registration dossier.
The ACTD consists of Parts I to IV which have subsections A to F whereas ICH – CTD has 5 Modules with subsections that are numbered. The administrative data of Part I is part of ACTD whereas Module 1 of ICH – CTD is purely country specific. The summaries of the quality (Part II), nonclinical (Part III) and clinical (Part IV) are located at the beginning of each part of the ACTD. The ICH – CTD dedicates these summaries in a separate Module 2. As the ACTD does not have such summary part, it consists of only 4 Parts and not 5. The main differences between the ICH - CTD and ACTDare listed below in Table II.
Table II: Overview of ICH – CTD and ACTD
|
DOCUMENTS |
ICH - CTD |
ACTD |
|
Administrative Documents and Product Information |
Module 1 |
Part I |
|
CTD Overview and Summaries |
Module 2 |
Incorporated in parts II, III & IV |
|
Quality Documents |
Module 3 |
Part II |
|
Non – clinical Documents |
Module 4 |
Part III |
|
Clinical Documents |
Module 5 |
Part IV |
CONCLUSION
Every country has its own Drug Regulatory Authorities, which is responsible to enforce the rules and regulations and issue the guidelines to regulate the marketing of a drug. Therefore, it is very difficult, especially for the companies with global approach to develop one single regulatory approach for a Marketing Authorization Application (MAA) for a new drug on the basis of one dossier submitted simultaneously to different countries in the world. It is very important to know in detail and exactly the regulatory requirements in each concerned country where a Registration Dossier should be submitted to establish a suitable regulatory strategy before the submission in order to avoid any major difficulties.
Regulatory standards in ICH countries (EU, USA and Japan) have been progressively tightened. They have developed a common for submission for MAA. All drug application submissions must be made in the CTD format. Non – ICH countries include all the countries outside the ICH. Non – ICH countries represent countries with low income and less developed regulations. They do not have sufficient technical and regulatory resources to comply with the ICH guidelines.
Therefore, due the variations in the regulatory norms in the Registration Dossier in different countries of the world, there is a strong need for harmonization either by the ICH or WHO as the Regulatory Agency for harmonized approval of drugs at the global level.
REFERENCES
1. archives.who.int/tbs/qual/s2300e.pdf (Accessed on August 22nd , 2011)
2. dictionary.reference.com/browse/dossierupport (Accessed on August 22nd , 2011)
3. apps.who.int/prequal/info_general/documents/WHO_DMP_RGS_98_5_R.pdf (Accessed on September 7th, 2011)
4. en.wikipedia.org/wiki/Marketing_authorization (Accessed on September 7th, 2011)
5. policycures.org/downloads/DNDi_Registering_New_DrugsThe_African_Context20100108.pdf (Accessed on September 20th, 2011)
6. ich.org/products/ctd.html (Accessed on September 30th, 2011)
7. en.wikipedia.org/wiki/Association_of_Southeast_Asian_Nations (Accessed on October 9th, 2011)
8. moh.gov.bn/pharmacyservices/download/ASEAN%20Common%20Technical%20Document%20(ACTD).pdf
9. ich.org/about/history.html (Accessed on July 1st, 2011)
10. ich.org/fileadmin/Public_Web_Site/ICH_Products/CTD/M4_R3_Organization/M4_R3__organization.pdf (Accessed on July 23rd, 2011)
11. ich.org/products/ctd.html (Accessed on July 25th, 2011)
12. aseansec.org/about_ASEAN.html (Accessed on September 10th, 2011)
13. wikipedia.org/wiki/Association_of_Southeast_Asian_Nations (Accessed on September 10th, 2011)
14. bpfk.gov.my/berita%20-%20berita/April%202001%20asean.htm (Accessed on November 6th, 2011)
15. pacificbridgemedical.com/services/regulatory/registration/others-drugs (Accessed on November 19th, 2011)
16. moh.gov.bn/pharmacyservices/download/ASEAN%20Common%20Technical%20Document%20(ACTD).pdf (Accessed on September 13th, 2011)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









