 About Authors:
About Authors:
L.D.Budania
Seth G. L. Bihani S. D. College Of Technical Education,
Institute Of Pharmaceutical Sciences & Drug Research, Gaganpath,
Sri Ganganagar, Rajasthan 335001
*ldbudania@gmail.com
ABSTRACT:
Stability is an essential quality attribute for drug products. If there is any functionally relevant quality attribute of a drug product that changes with time, this evaluation checked by pharmaceutical scientist and regulators who quantify drug product stability and shelf life. The rate at which drug products degrade varies dramatically. E.g. radiopharmaceutical products. Since the evaluation of the stability of drug is highly specialized and esoteric nature. Drug stability concerns about drug product safety, efficacy, and quality, found it to appropriate. Stability studies are done through the regulatory agencies such as FDA and HPB (health protection branch).
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1527
1. INTRODUCTION
It was at the time of manufacture, and more importantly, it may not meet the minimum required for efficacy. For a solution, a precipitate may have occurred. This may not affect the chemical content, but for a parenteral product it would, obviously, be quite unacceptable and for an oral solution it would also be unsatisfactory, because the dispensing pharmacist would rightfully question the integrity of the product. The caking of a suspension impairs the dispensing of a known amount of drug in a teaspoon, and a separated or broken emulsion or cream obviously will not have the same emollient properties as would a proper product. Physical stability will be treated by product category in the same order as in the case of chemical stability.The formulation is totally unchanged throughout its shelf life and has not suffered any change by way of appearance, organoleptic properties, hardness, brittleness, particle size etc.
Physical degradation may change their pharmacological effects, resulting in alter efficacy therapeutic as well as toxicological consequences. Because pharmaceuticals are maintain their quality until the time of usage or until their expiration date. The most easily understood and most studied form of drug instability is the loss of drug through a chemical reaction resulting in reduction potency. Loss of potency is a well- recognized cause of poor product quality.
[adsense:468x15:2204050025]
2. PHYSICAL STABILITY OF SOLUTION
Solutions are broadly divided into two categories: oral and parenteral solutions. Appearance, in both cases, is an important factor. In the case of oral solutions, organoleptic properties are also of great importance. Organoleptic evaluation is usually done subjectively, i.e., a tester (operator, technician), will judge the product and score it, either numerically or descriptively or both. In the case of appearance of solutions, there should always be a subjective statement (quantitative or subjective description) even if more quantitative instruental parameters are recorded. A few words are therefore in order regarding organoleptic and appearance testing.
A. ORGANOLEPTIC TESTING
For organoleptic testing it is important to establish a test panel early in the stability program. (or if a stability program is in place, but no such testing is carried out, a test panel should be selected at the first opportunity when a product with important taste or odor properties is placed on stability) Many companies utilize just one tester for the task of organoleptic testing, but this can be shortsighted, because the tester may leave, go on vacation, or become ill, and in that case the logical solution is to assign someone else to the task. There may be an evaluational bias between the two testers, and this should be established at the onset. First of all, the depth of organoleptic capacity should be tested. This can be by asking the tester to taste serial dilutions of a bitter substance (e.g., quinine). e a sensitivity level can be established.Acontrol of e.g. water or high dilutions should always be part of the protocol. It should be noted that the technicians are not taste testers in the ordinary sense. That is, it is not necessary to match their “likings” to that of the general public. Rather, it is important that they can (a) duplicate their results and (b) remem- ber them, since they will be asked to taste a preparation that they originally tested 3 or 6 months earlier. In so doing they would have to score the degree of flavoring, e.g., is it less than originally present, i.e., is the flavor being lost? They would also have to be able to describe the flavor well originally. For example, if the chemical is slightly anesthetizing, the duration of the anesthesia would be important. If there is interaction with a plastic bottle, are off flavors appearing in the product? Finally it is important to screen several testers to ascertain that they give the “same result.” In describing the flavor, several categories can be used (degree of sourness, degree of saltiness, level of flavor, type of flavor). Each of these may be assigned to a level.Aflavor profile may hence be established, and this can then be reestablished at several time points in the room-temperature storage. It is not recommended to evaluate results from higher temperatures (although they may be carried out).
In aquous solubility of a drug substance is a fundamental property that should be evaluate early discovery. Lack of solubility can effect efficacy and toxicological relevant exposure in animal.this characteristic will also affect the future developability of the formulation efforts for the compound.solubility depend upon salvation energy solvent.in the solvent overcoming both the crystal lattice energy of the solid and the energy of create space in the solvent for solute. Thus the solubility of compound depend not only properties of the drug molecule itself such as polarity, lipophilicity , ionization potential and size but also on properties of the solvent and solid throughout discovery range from the method that dilute dimthylesulfoxide stock solution in aquous buffer and mimic the method in which high throughout assay are run, to those measuring psedoequilibrium solubility using crystalline solid and aquous buffers. Detection methods include turbidimetric method , uv plate readers, liquid chromatography (LC)/uv and LC/MASS spectroscopy. The solubility method chosen depend upon the desired time, quantity of compound and the quantity of compound and quantity of results requires.
The factor affect as-
(a) Dielectric constant:
The rates of degradation between ions and dipoles in solution depend on the bulk properties of the solvent, such as the dielectric to a variation in rate constant with change in dielectric constant. For example ion depolarization rate constant have been related to dielectric constant D of the solvent through in k?
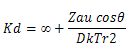
Where the kd that is rate ?∞ is the rate constant at infinite dielectric constant , ZA, u and r are ion charge , dipole moment and the shortest ion dipole distance, respectively and k is the boltazmann constant. The term ?represent the alignment of reactant and cos?is unity in the case on aligment. Thus as the dielectric constant increase , the rates of anions , dipole reaction decrease and the rates of cation dipole reaction increases. As indicated by linear relationship with positive slop in log k verses 1/D plots, the hydrolysis rate constant for chloramphenicol in water propyl glycol mixture increase with deceasing dielectric constant, suggesting a hydronium ion dipole reaction, whereas in alkali its anionic form is degraded by hydroxide ions. The dipole- cation reaction at PH 1 exhibit log versus 1/D Plots with a negative slope, suggesting that reactant alignment is opposite to the head on alignment. The observation that the rate of anion , anion reaction at pH11 is independent of dielectric constant has been explained by assuming that a change in the bulk dielectric constant is not reflected in the microscopic dielectric constant and no effect on the reaction.Effect of change in solvent diectric constant on the degradation rate of chloramphenicol.
(b) VISCOSITY
Viscosity of solution serve the palatability or improve portability. This can be achieved by increasing the sugar content in the syrup or adding viscosity controlling agents , such as polyvinylpyrolidone and various cellulose derivative like methyl cellulose or carboxy methyl cellulose.
(c) Taste ( flavour )
Mostly combination of flavouring agent are used in industries. Apart from this, methanol and chloroform also used as desensitizing agent because they provide the odour to preparation along with some local anesthetic effect. In food industries the monosodium glutamate mainly used. The change flavoring agent can be determined by vapour phase chromatography.
(d) Colour
Colour is measured by spectrophotometrically. Clarity measured by passing the beam of light on sample solution and measured scattering. Turbidity is measured by turbidometry.
(e) Integrity of container
Some plastic container may shrink when contact with the preparation or may cause corrosion of cap. Sometime the glass container may change the pH of solution and may affect the stability of preparation. By investing the intrinsic stability of the drug it is possible to advise on formulation approaches and indicate type of excipient, specific protective additives and packaging which are likely to improve the integrity of the drug and product. As various pharmaceutical dosage form present unique stability problem, they are discussed under.
B. SUBJECTIVE APPEARANCE TESTING
Solutions, particularly parenteral solutions, may have a tendency to discolor slightly. 0ften it is not possible, within analytical sensitivity, to establish either the source of e color or the level of the substance causing it, In this case it is a good practice to use a color standard to describe the “intensity” of the discoloration. For instance, uses the so-called Roche Color ~standard (RCS), which uses pound (the identity of which is a secret) that can be reliably reproduced and has exceptional color stability. Making up serial dilutions of this solutions of different “slight” discolorations; they are denoted so that a solution can always be compared in this fashion, old-fashioned Dubuque colorimeter (which can be used with advantage in this type of situation).
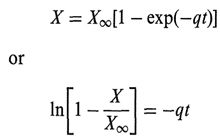
Where q is a constant, t is time, and XW is found by iteration. This allows (from accelerated studies) a visual estimate of the worst appearance that a product could take on.
(a) PARENTERAL SOLUTION
In Parenteral solutions, physical stability includes interaction with a container and changes in chemical composition that give rise to physical changes. For instance, may discolor slightly without showing detectable changes in content of parent compound. Such discolorations can be followed as described immediately above, and at times they are detectable analytically. They are often oxidative in nature and metal ion catalyzed. Such a case in captopril .It has reviewed the stability aspects of parenteral products coloration is often either photochemical or oxidative. Has summarized the usually used antioxidants and chelating agents.
SWIRLY PRECIPITATION
Often a parenteral solution will develop a swirly precipitate upon storage. This is Most prevent in vials and is usually an interaction with either the glass or stopper.it may be difficult for the uninitiated to detect such slight changes, and reason to use for this type of evaluation is a parenteral inspector. It is difficult to estimate the extent of the precipitate; it can be done by mechanical counting (e.g., with a Coulter counter), but the results are difficult to interpret, Often the count pond to the “severity of the swirl.” re to the point is how many a box of e.g. 144 vials is placed o S type of stability, then the vials can be examined from time to time, and one may establish how many vials have become swirly. This number be treated in proper fashion to evaluate the severity of the problemasmentioned, the occurrence of swirls is usually a container interaction, and a change in the stopper or the glass may often eliminate the problem. Vials should always be stored (a) upright, (b) on the side, and (c) upside down to check the inter- action with the stopper. In this way primary evidence can be established as to the culpability of the closure.
WHISKERS
It occurred at the tip of the ampoule in a large percentage of ampoules upon room-temperature storage. This is a defect that will occasionally occur in a product. It is due to pinholes in the glass. The solution wicks out, and the liquid evaporates on the outside. The solid that is formed serves to wick out more solution, and long crystals or “whiskers” may occur. One might ask why the pinholes have not been detected in the dye test used for autoclaved ampoules. There are two reasons. One is that the hole may be too small for detection (about 0.5pm is the detection. limit). The other is that the ampoule was tight at the time of manufacture, but the heat sealing line was run too rapidly, or the flame temperature was incorrect, so that the glass did not have time to anneal pro- pearly, and the strain caused the crack during storage (not immediately after manufacture).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CLOUD TIME
Sometimes a cloud will appear in a product as the storage time progresses, and this is most often due to chemical changes in the system. If for instance an ester (e.g., polysorbate, which is a fatty acid ester) hydrolyzes, then the produced acid may be poorly soluble. If the solubility is denoted S, then the following holds: If the reaction in general is written
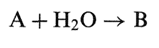
Where A is a drug of initial concentration A0 and is the decomposition product with ility S (which is assumed to be limited.
Arrhenius plotting. Such plotting is quite predictive, The precipitation may also occur by the solubility product being exceeded, or from any situation leading to a product with limited solubility. There are other causes for precipitation on storage, one being the original use of a metastable form, so that the solutions in question, in fact, are super- saturated solutions. It was the author's experience, at his tenure at Hoffmann-la Roche in 1965, that a product to be introduced (Taractan Injectable) was in this category. Several pilot batches had been successfully made, but the first production batches precipitated, a more stable polymorph crystallizing out, This necessitated reformulation to a lower strength (corresponding to the lower solubility of the stable polymorph) and subsequent resubmission of data to the FDA. This points out the importance of careful preforrnulation studies of the solubility of compounds. Errors of the above type are costly, both in terms of resubmission and in lost market time. Even official products fall into this category.
The viscosity of these agents is often Ingham bodies, i.e., they posses a yield value. The correct way of checking the is, therefore, with e.g. cup and bob viscometer, so that a rheogram can be drawn. In this fashion it is possible to check both changes in yield value and slope of the rheogram (apparent viscosity). For very liquid solutions (dilute aqueous solutions) this is difficult, and most often it is best followed by the use of an stwald-Fenke pipette two pipettes (with different flow should flora time should be used in is case, because the once in the measured vise erasure of the yield value (although calculation of the yield value from the difference is a priori not not yield value and apparent viscosity are functions of concentration t al., 1980); in a multicomponent system~ there will usually be one moment responsible for viscosity, and it is the breakdown of this one compound that would be of importance. often when drastic occur in viscosity, bacterial contamination can be suspected. precipitation is tied into solubility, as seen in the foregoing.
(b) ORAL SOLUTIONS
The main types of changes in appearance of oral solutions (syrups,elixirs,etc) are loss of dye, precipitation, and bacterial growth. Precipitation has already been dealt with to some degree, but some cases particular to oral solutions will be mentioned. Change in dye content will be treated below.bacterial growth will be treated separately.
3. PHYSICAL STABILITY OF DISPERSE SYSTEM
Disperse systems are suspensions and emulsions. The rationale for the Physical tests carried out on these will be discussed below.
A. SUSPENSION
It would be desirable to have a suspension that did not settle (and there are such suspensions), but the general rule is that a suspension will settle, and therefore there are two parameters that are followed in this respect, namely sedimentation rate and sedimentation volume. When the sedimentation volumes are small, then there is a tendency for the suspension to cake, and hence various types of shaking tests are carried out. Tests can be purely subjective, in that a tester notes that e.g. the suspension after three months’ storage at 25°C was “difficult to resuspend, leaving some cake at the bottom,” Such subjective tests should always be included in a pro but more quantitative means are desirable also. Atypical quantitative test is to rotate the bottle under reproducible conditions. The type of setup used for solubility determinations is a good type apparatus for this purpose.
One way of accelerating the settling is to place the suspension product on a shaker at e.g. 37°C. This makes particle movement more rapid and allows the fine particles to slip into the interstices of the larger particles, hence promoting a close packing. This can then be used to judge qualitatively whether caking will take place. It might be thought that centrifugation would be a good way in which to ‘‘accelerate’~ sedimentation, and the Stokes law indeed predicts this. However, it gives only an acceleration of the “initial settling rate,” and the further settling, and the caking phenomena in which the formulator is interested, are not well predicted by this method. Some caking is due to crystal growth, and this is accelerated by the use of freeze-thaw tests, i.e., alternating the temperature every 24 h from e.g. 25°C to -5°C (or some other low temperature above the freezing point of the product). The tem- prelature cycle will promote crystal growth, and the effect of this on the product can be assessed. The freeze-thaw cycle has the advantage of emulating (and overstating) some real conditions to which the product could be exposed during shipping. Zapata et al. (1984) have described the effect of freeze-thaw cycles on aluminium hydroxycarbonate and magnesium hydroxide gels. Coagulation after freeze-thaw cycles led to the formation of aggregates that were visible. These aggregates were particles in a primary minimum, and these were only reseparable by ultrasonic treatment. The freeze-thaw cycle affected content uniformity of both the gels, but the treatment did not alter the surface characteristics or the morphology (as judged by x-ray powder diffraction). It cause a reduction in the acid neutralization rate, and the rate of sedimentation increased. The effect was pronounced after the first cycle (and indeed most of the effect occurred at this point). The duration of freezing was not important, but the aggregate size grew inversely with the rate of freezing. The use of polymers in the suspensions reduced the effects of the freeze-thaw cycle.
Freeze-thaw cycles (aside from being a stability monitoring tool) can be used to screen products as well, the best of a series of suspensions or emulsions being the one that stands up best to the test. This on the surface may be logical, but without a theoretical basis it is difficult to judge the generality of such a statement.
B. SEDIMENTATION VOLUME
If a suspension is particulate, then the particles will (approximately) settle by a Stokes law relation, i.e., the terminal velocity, v, is given by
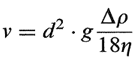
where the constant g is gravitational acceleration, A p is the difference in densitybetween solid and liquid, q is the viscosity of the liquid, and d is the diameter of the particle. The final apparent volume of the sediment, provided it is monodisperse, would be given by the fact that in cubical loose packing a sphere of diameter d will occupy the space of its confining cube, i.e., the sedimentation volume will be
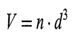
where n is the number of particles per cm3 of suspension. Since their density is p g/cm3, then (denoting the dosage level Q g/cm3) the following holds:
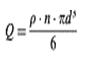
so that, solving for n,
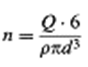
which inserted in gives
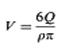
Hence it becomes difficult to separate them, and the precipitate becomes a cake. This would prevent redispersion by shaking and would make proper dispensing impossible. It is a for- mulation goal to prevent this from happening, and this is done by adjusting the zeta potential, as will be discussed shortly. From a formulation point of view, it is better to have the particles at larger distances, e.g., in the secondary minimum occur- ring at longer distances. Adiscussion of the connection between caking tendency and the so-called zeta potential is beyond the scope of this book. Suffice it to state the following: particles are suspended in a liquid, they acquire a charge (and the liquid acquires a similar opposite charge, to maintain electroneutrality). The zeta potential is related to this charge, and caking is prone to happen if the charge potential is outside a range of -10Mvto +10 mV. If the zeta potential is high it can be lowered by the addition of negatively charged ions. Highly valent ions (e.g., citrate) are preferable. On the other hand, if the zeta potential is low, then it can be increased by the addition of positively charged ions (e.g., aluminium ions). The zeta potential is measured with a zeta meter, In this the particles are placed in an electrical field (between two electrodes, the voltage of which can be adjusted), they are tracked under a microscope, and their velocity is determined. The relation of velocity to voltage allows determination of the zeta potential. It is worthwhile occasionally to check the zeta potential in a stability check of suspension (and emulsion) products.
The zeta potential is close to zero, the suspension will be flocculated, i.e., the particles are positioned in the secondary minimum. The floccules are large and hence settle more slowly, but on the other hand the sedimentation volume is large. Since the particles are in the shallow minimum (small potential, i.e., easy to disrupt), they are easy to resuspend.
There are suspensions that do not settle. Here the yield value of the suspension is so large that the gravitational force does not exceed it. In this case it is very important to carry out complete rheological profiles at different time points in the stability program, to insure that the yield value is not changing. In such a system the yield value is a function of the solids content and the viscosity of the medium. If the viscosity imparting substance deteriorates, or if the flocculation characteristic (the “diameter” of the particles) changes, then the yield value may change, and what originally was not prone to cake might at a later time have such a propensity. It has been stated elsewhere that for Ingham bodies, a yield diameter of the bottle can be calculated and below this bottle diameter there will be no settling.
C. TEMPERATURE TESTING OF DISPERSE SYSTEM
A suspension is, as the name implies, a two-component system consisting of a solid and a liquid phase. (Gas phases are considered nonessential in this connection). Obviously, the solubility of the compound is a function of the temperature, and at a given temperature above 25°C this solubility will be reached. Testing about this temperature obviously has no meaning as far as suspension stability (neither physical nor chemical) is concerned. Prior to starting a program, this temperature should be established, so that unnecessary sampling stations can be avoided.
D. SEMISOLID SUSPENSION SYSTEM (OINTMENT, SUPPOSITORIES)
Some semisolid systems (ointments and suppositories) are suspensions. Their testing is not different, in general philosophy, from what is described above, except that the archeology is checked differently.
The factors checked for in stability programs of such products are the following:
1. Consistency fell to the touch
2. Viscosity
3. Polymorphism
It is mentioned elsewhere that migration of a “disperse” phase within a semisolid product is quite possible when another phase is present. This situation may occur in the case of the use of benzocaine in, for instance, a suppository wrapped in aluminum foil coated with polyethylene. Polyethylene lining of aluminum wraps of suppositories is used to prevent contact between the metal and the suppository, and in most cases this has a positive effect. However, a partitioning of drug or additive between the two phases may be possible if the drug or additive is suspended in the suppository. Denoting its solubility in the polyethylene S, and the solubility in the suppository base Ss, the compound would disappear from solution in the suppository at a rate proportional to Sp - Ss, and “disappeared” compound would be replenished by dissolution from the solid phase. The rate of disappearance would be governed in that the value of Sp would increase by a sigma minus relation (i.e., in the same manner as the appearance of decomposition product in a first-order reaction), and this then would be the over- all “loss” of compound as a function of time. Since this is a first-order overall relationship, the “decomposition” would, initially, appear to be first order.
E. OINTMENT AND TRANSDERMAL
Polymorphism can be followed by x-ray analysis and in some cases by thermal methods. There is, in fat systems, the possibility of trans etherification, and this can be tested for chemically. The problem of morphology changes is often of particular importance and of particular frequency in the case of suppositories. In this type of product, it is also important to check for migration of suspended/dissolved substances. Often a sub- stance is added to a suppository as a suspended particle, which is soluble in the suppository base to some extent. The phenomenon of dissolution will, of course, become evident by checking the particle size as a function of time. If a substance is soluble in the base, then it is preferable (if possible) to saturate the base with it at the onset. For this reason it is necessary to determine the solubility (S gm/gm) of the drug (or other) substance in the base. A Van’t Hoff plot [solubility as a function of temperature (T’K), i.e., plotting ln [q versus 1 /U will allow extrapolation to room temperature. In manufacturing it is advisable to dissolve the drug (or other substance) to the extent of its solubility during the intermediate temperature phase of manufacturing (where the preparation is still quite fluid) and then suspend the rest at a lower temperature. An example is ascorbic acid, which is a good antioxidant in Carbowax bases. To exert its antioxidant action it must, however, be dissolved (and it is quite soluble in polyethylene glycols). Dissolved drug (or other substance, e.g., benzocaine) will diffuse in the suppository base, and can, for instance, partition into polyethylene linings of the suppository wrap.
Release rates are important in many topical preparations in particular in transdermal preparations. Here there are several investigational methods available.
In-vitro methods involve placing the ointment on a membrane and Measuring the appearance of drug in a receptor compartment on the sink side of the membrane. oelgaard and Mollgaard (1983) have, for instance, described the in-vitro release of linoleum acid through an in-vitro membrane. They mounted abdominal human skin in one case and skin from hairless rats in another to open diffusion cells. The dermal side was bathed with a rece tor medium stirred at 37°C. The medium was 75 mL, of 0.05 N phosphate buffer ”-7.4) which contained 0.05% ic F68 and 0.01% butylhdroxytoliene, alter two ingredients added in to increase the lipid solubility. Linear, Fiction diffusion curves were obtained. In a stability program, such tests are obviously useful and should be repeated periodically, but an “internal standard” or “calibrator” should be use i.e., a stable test substance, the diffusion of which is known (e.g., salicylic acid). their pseudo-in-vivo methods involve shaved or hairless rabbits, or cadaver skin. The interaction between ointment and container (patch) should also be part of the stability program. Some of the testing applicable to semisolid emulsion systems is also applicable to ointment systems and will be discussed at a later point.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
F. THE EMULSION INTERFACE
The factors that stabilize the emulsion system are a layer of surfactant and protective colloid on the exterior of the droplet. The amount of these two must be such that they separate
cover the entire area of the droplets, otherwise coalescence will occur to the extent that the area, A, of the droplets will be reduced to such a point that it now will be completely covered by surfactant and protective colloid. If, for instance, 1 g of emulsion contained Wg of droplets of a size d pm and the oil had a density of p g/cm3, then there would be n droplets per cm3, where n is given ach particle has a surface area of d2, so that the total area is
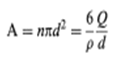
G. EMULSION TYPE
In emulsion formulation, the type of emulsion is of concern. If it is desired to make an oil-in-water emulsion (olw, i.e., oil is the discontinuous phase), then it is important that phase inversion not occur. Investigating this possibility must be a task in the stability program (and is usually carried out by the formulator, not the preformulator)most often phase inversion is associated with creaming and separation and will be noticed in the appearance testing of the emulsion. Such phenomena lead to graininess of feel. In so e cases part of an emulsion will invert, another not, and then there is a distinct difference in appearances in various regions of the the possibility for inversion should always be considered. It is the more likely the closer the system is to a close-packed system of spheres. In this connection, their of the formulator9s tasks should be to determine the inversion temperature. is is at times used to advantage in the manufacturing step, in that, in producing the emulsion, the inverse emulsion is produced at high temperature; this is then cooled, and at the inversion temperature, the “correct9’ type will result. on version in this manner gives rise to very small globules, and homogenization is then often f an inversion temperature exists, then accelerated testing above . So preliminary testing is always advocated, if accelerated, the philosophy being that there is no sense in testing a system above a temperature where it converts to a physical state that differs from that at room temperature (or recommended storage temperature).
It shown that there is a correction between phase inversion temperature and the rate of coalescence . is possible to use a combination of sedimentation of fractionation and photon correlation spectroscopy to record droplet sizes in fat emulsions, and this would appear to be an excellent technique for studying the coalescence of finer spheres, and hence to obtain an extrapolator tool early on in the storage of an emulsion system.
H. BREAKING AND COALESCENCE
It can be concluded from what has been mentioned that the reasons for breaking would include Chemical incompatibility between the emulsifier and another ingredient in the emulsion system (Borax and gum acacia is a case in point) Improper choice of surfactant pair (e.g., wrong High electrolyte concentration Instability of an emulsifier Too low a viscosity Temperature.
As shown in the foregoing, breaking and creaming of emulsions are the typical defective criteria to be looked for in stability programs. Breaking implies that the emulsion separates into two distinct phases It is a slow process, it often manifests itself in the appearance of small amounts of oil particles on the surface, and it then is referred to as oiling. When separation into two emulsions occurs (as described above), then the phenomenon is called creaming. A rapid test for this is to dip a finger into the preparation and notice if there are different “colors” present rown, 1953). Also, a creamed olw emulsion will not drain off the skin with ease, and the converse holds for a creamed wlo emulsion. A few words regarding the effect of ionic substances and the actual process of flocculation and coalescence are in order. Vanden Tempe1 (1953) demonstrated that flocculation and coalescence are two different processes. Flocculation depends on electrostatic repulsion (and is akin to the zeta-potential considerations discussed previously). Coalescence depends on the properties of the interfacial film.
Cations, as a whole, are less soluble in the oil phase than anions, and this gives rise to negatively charged droplets (akin to the creation of a zeta-potential in suspensions). The potential drop over the film depends on the nature of the electrolyte (and it should be noticed that there is a diffuse double layer in both liquids as opposed to the case of suspensions, where there is only one diffuse double layer).
Electrolytes may either improve or worsen the stability: If they eliminate the protection offered by the surfactant /protective colloid system then coalescence ost often electrolytes have the effect of reducing the emulsifying powers of surfactants and causing salting out or actually precipitating the surfactant. However, in some cases, electrolytes will favorably affect the potential drop over the two double layers, and in this case they may stabilize the suspension system.
4. PHYSICAL STABILITY OF SEMISOLID DOSAGE FORMS
Semisolid emulsions (cold creams, vanishing creams) are not different, in general philosophy, from the above, except that the rheology is checked differently. Davis (1984) has reviewed sophisticated means of checking the stability of these types of systems. He lists the following properties as being important in stability programs for semisolid emulsions:
1. Particle size
2. Polymorphic/ hydration/solvation states
3. Sedimentation/creaming
4. Caking/coalescence
5. Consistency
6. Drug release
Of these, particle size, sedimentation/creaming, caking-coalescence, and consistency have been discussed earlier. Following viscosity as a function of time is here of particular interest, The problem is how to measure the viscosity, and what viscosity in essence means. (1987) points out that changes in viscoelastic properties are much more sensitive than simple continuous shear measurements (Barry, 1974). He demonstrates this via data published by Eccleston (1976). Here the variation of the dynamic viscosity (q) and the storage modulus (4) are shown and compared with the same type of graph for apparent viscosity (p’) from continuous shear experiments. It is obvious that the two former measurements are much more sensitive.
A. TRANSDERMALS
The most important concern about transdermals is the release of drug substance from them and the stability of this property. Other properties (stickiness, appearance, etc.) are of importance as well, but the release characteristic is paramount. Kokobo et al. (1991) have described a means of checking this in vivo by using a single diffusion cell. it have reported on the interaction between primitive adhesives and drug combinations used in transdermals. . The data fit neither a diffusion equation (In of retained versus time) nor a square root equation directly. It would appear that if one allows for either an initial dumping in the diffusion equation (or includes more than one term arrear equation) or a lag time in a square root equation, then the data will
B. ACCELERATED TESTING AND PREDICTION
Accelerated testing of physical properties of disperse systems is not as clear-cut as for instance chemical kinetics prediction. For instance, the stability of properties of semisolid materials is very difficult, for instance, for creams and ointments that give rise to bleeding there does not seem to be any reliable predictive test. Yet a series of stress tests are used for disperse systems. They include Shaking tests centrifugal tests Freeze-thaw tests.
For the freeze-thaw test, the question is what the minimum temperature should be, temperatures from -5” to +5”C being the most common. -5°C frequently gives rise to phase separation and irreversible changes that would not be seen in usual temperature ranges (Nakamura and Okada, 1976), but again, such tests may be used to select a “presumably best” formula from a series of preparations in product development. Results of a typical freeze-thaw . centrifugation has been used by some investigators . The general idea is that g can be increased city predicted by Stokes’s law , but often the stresses caused by centrifugation may cause coalescence, which would not occur during nor- mal collision stress. Some investigators claim fair success in predictions by this means, but as avis (1987) cautiously states, “as a general rule it can be stated that systems that accelerated stress conditions should be stable under normal storage con- however the corollary is not necessarily true.” That is, if the preparation fails the test it may still be all right, but if it passes the test it should be all right. although this may be true overall, one can visualize that if a preparation is centrifuged right after manufacture, then the stress does not include the chemical changes (surfactant decomposition for instance) that occur on storage, and in this it may give too optimistic a prediction. usual et al. (1979) have measured phase separation at several different centrifugal gas and have established from these data a so-called coalescence pressure. This (again recalling that the test does not account for chemical changes on storage) may be an appropriate parameter. One predictive method in formulation is the correlation afforded by coalescence rates , and this is rational in selecting the “best,, of many formulations; in general the system with the highest phase inversion temperature is the best. The (nonchemical stability dictated) coalescence rate could theoretically be calculated prior to storage, and the difference between observed and calculated then attributed to chemical stability causes.
For emulsions, it should again be pointed out t t rapid creaming and necessarily mean rapid coalescence. that attempted tie zeta-potentials to emulsion behavior on storage, but the generality of such an approach has been test is usually carried out , and the Philsoppy here is to intensify the collision frequency between globules.
5. PHYSICAL STABILITY OF POWDERS
Pharmaceutical powders are for reconstitution into either suspensions or solutions. A prescription example of the former is chloramphenicol palmitate, where the arried out by the pharmacist prior to dispensing. An example tamucil, where the customer reconstitutes the product of solutions are AchromycinFM(which is a parenteral the-counter examples of oral solutions of this type are older produ gna Granules (LederleTM). Analogies in the food area are fruit hich are sold in packets and reconstituted by the consu~er to a certain volume. The main physical concerns in this type of product are appearance, organoleptic properties, and ease of reconstitution. nly the latter will be treated here. There are several reasons a powder may change dissolution time as a function of storage time. The most common reasons are (a) cohesion, (b) crystal growth, and (c) moisture sorption, which causes lumpingup of powders. The latter is simply due to the dissolution and bridge-forming that occurs and is akin to what happens in wet granulation.
There are two situations in whichOne is due to the polymorphism. the original product is either a metastable polymorph or amorphous, the conversion may occur in storage. For this to happen, some stress, e.g., the presence of moisture, must occur. The stress need not necessarily be moisture, conversion of a small amount of powder might occur in the filling head of the filling machine and then propagate in time. If the content of the drug substance is such that there are no neighboring drug particles, then this conversion is limited. Particularly, contact points allow for propagation of conversions in situations where the spontaneous nucleation prob- ability is low. The presence of moisture will accelerate conversions of this type, once a seed of the stable polymorph (or in the amorphate situation, once a crystal) has formed. Crystal growth is, per se, not to be expected. It is true that, by the Ostwald-Freundlich equation, a larger crystal is thermodynamically favored over a smaller one; but the energy differences in the usual particle ranges is small and the activation energy high, so that the likelihood is rather low. If sufficient moisture is present so that the vapor pressure in the container exceeds that of saturated solution, then some of the drug will dissolve in asorbed moisture. Fluctuations in temperature are never absent and would cause dissolution followed by precipitation, and this can lead to crystal growth. In cases where a drug substance is capable of forming a hydrate, and where an anhydrate is used, growth by way of hydrate formation is possible. Ease of reconstitution is usually carried out subjectively, in that a tester carries out the reconstitution in the prescribed manner and records the length of time required to finish the operation. For this purpose it is important to have detailed directions on how the reconstitution is to be carried out, and to be sure that there is no operator-to-operator performance bias. To insure the latter, a set of operators is usually selected for the operation at point in the stability history. These operators will then be the test instruments for all testing of reconstitutability of oral powders. The manner of screening operators could be as follows.Arandom sample is taken of a batch of a product. Random sets of four are taken from this random sample, and e.g. three operators tested. They are each given four samples to reconstitute on the first day, four on the second day, and four on the third day. It is a good policy to have two batches and mix them by day and operator, so as to carry out the test in a blind fashion. The results of such a screening.
As mentioned, the most common reason for increases in reconstitution time upon product storage is that the powder becomes more “lumpy” through cohesion developing over time or because it becomes coarser due to crystal growth.
(a) PARAMETER
The physical properties associated with tablets are disintegration, dissolution, hardness, appearance, and associated properties (including slurry pH). For special tablet products (e.g., chewable tablets) organoleptic properties become important. These have been described earlier, but in the case of tablets, the chewability and mouth feel also become of importance. The properties will be discussed individually below.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Tablet Hardness
The “hardness” properties of a tablet are usually assessed by subjecting the tablets to a diametral failure test. The tablet is placed between two anvils, one of which is stationary. The other anvil is moved at constant speed against the tablet, and the force (as a function of time) is recorded. The force, at which the tablet breaks is denoted the “hardness” and is usually measured in kp (kilopond= kilogram force). Other older units (Strong Cobb Units, SCU, or pound force) are used, usually when older instrumentation is used. Until recently, one limitation was that forces over 20 kp would simply register as F > 20 kp. Newer instrumentation allows for quantitation of higher forces. From a stability point of view this is important, since the better a parameter can be quantitated, the clearer the picture that emerges will be.
For good formulations, this maximum does not occur until very high pressures (outside the range of pressures used in pharmaceutical tableting). The maximum occurs because above the critical pressure, P*, the tablet will laminate or cap, and a laminated tablet will contain strata of air and hence be thicker and weaker. Tablet thicknesses will respond in a manner opposite to the hardness, i.e., show a minimum (e.g., at 500 MPa . The reason for this phenomenon is the following: As applied pressure increases, the number of bonds, N, increases as well. ut assuming that there is a number of bonds, N*, that can be formed, then the strength, H.
Its side from the quoted instance of porosity changes and expansion, there are cases where crystallization of a soluble compound has occurred via the sorbed amounts of moisture in the tablet, This happens most often with very soluble compounds, and in such cases it is important to ascertain storage in a dry environment.
A test that is now a requirement in the ICH Guidelines is storage in the final container at 40°C, 75% RH. During this test moisture is usually adsorbed by the tablets, and this can then cause softening of the binder bridge because of moisture uptake. At times, redrying will reinstitute the original hardness, Sometimes hardening occurs when the asorbed moisture causes recrystallization of a compound or Sexcipient.
Disintegration
Tablets (whether coated or not) are usually subjected to a disintegration test. The disintegration was the first in-vitro test used by the U.S.P. It is now not obligatory compendially (but is recommended); in an obligatory sense it has been replaced by the dissolution test. This latter, hence, is the more important test, but it will be seen that there often is a correlation between the two, and since the disintegration test is much more easily carried out, a stability program will check disintegration frequently, and dissolution less frequently, primarily due to labor intensity. The apparatus used (U.S.P. XX, p. 958) is shown schematically in Fig. 22. It is an apparatus where six tubes are placed in holders on a circular screen, which is then raised and lowered between 29 and 32 times per minute through a distance of 5.3-5.7 cm in a 1000 mL beaker containing the disintegration medium (either









