ABOUT AUTHOR:
Hargobind Rajput
Punjab Technical University,
Jalandhar
hargobind.rajput@gmail.com
ABSTRCT:
The use of antiangiogenic drug is an advantage as a novel treatment for a number of conditions, ranging from cancer to psoriasis. Antiangiogenic therapy becomes a new course of treatment for cancer. This has led to the reassessment of antiangiogenic therapy for cancer, and new strategies have been proposed to increase the efficacy of these agents in this setting. Angiogenesis has also been implicated in other conditions that are notoriously difficult to treat, such as arteriosclerosis, arthritis, psoriasis and diabetic retinopathy.Increased understanding of the angiogenic process, the diversity of its inducers and mediators, appropriate drug schedules and the use of these agents with other modalities may lead to radically new treatment regimens for many of these conditions. The role of angiogenesis in different pathological settings and emerging antiangiogenic agents currently in preclinical and clinical studies are discussed in this review. However, while potential benefits are profound, limitations of antiangiogenic therapy have also been identified, suggesting that there is also a need for caution in applying these compounds to the clinical setting.
REFERENCE ID: PHARMATUTOR-ART-1835
1.1 INTRODUCTION
ANGIOGENESIS
The term “angiogenesis” was first coined by Dr. Arthur Tremain Hertig in 1935 in order to describe the formation of new blood vessels in placenta in monkeysand is defined as the formation of new blood vessels from preexisting vessels, capillaries and post capillary venules. Angiogenesis is important for the survival and growth of all cells and tissues, because the transportation of gases and nutrients in the blood through the vascular network is highly dependent on angiogenesis. [3] Angiogenesis is therefore, an incredibly beneficial phenomenon for many normal physiological processes such as normal tissue growth, embryonic development, wound healing and menstruation. [4]The improper angiogenesis in the body can lead to many severe disease states, e.g., inadequate angiogenesis can lead to ischemic tissues (tissues with restricted blood flow) and cardiac failure, while abnormally high levels of angiogenesis can result in pathological processes such as: cancer, age-related macular degeneration, atherosclerosis, rheumatoid arthritis, Crohn’s disease, diabetic retinopathy, psoriasis, endometriosis and adiposity [5].
1.2 MECHANISM OF ANGIOGENSIS
Blood vessels are joined with endothelial cells which are in direct contact with blood. Below theendothelial cells, blood vessels are surrounded by pericytes.Endothelial cells are active and selectively permeable to small peptides and proteins[6]. Angiogenesis is initiated by the release of pro-angiogenic factors which activate signaling cascades. The increased release of pro-angiogenic factors is normally in response to the release of cytokines by cells in a hypoxic or ischemic environment. Endothelial cell firstly initiate angiogenesis in all situations. The vascular endothelial growth factor (VEGF) is the most important molecule involved in the initiation of angiogenesis and cause vasodilation by releasingNitrous Oxide [7]. Increased endothelial cell permeability allows plasma proteins to enter the tissue and form a fibrin-rich provisional network to support the growth of new blood vessels [8].VEGF?s importance in initiating the angiogenic cascade is supported by the fact that its production is controlled by hypoxia inducible factor [9]. Even though VEGF is arguably the most important factor involved in angiogenesis, evidence has shown that angiogenesis in not entirely VEGF dependent [10]. In the process of angiogenesis, activated endothelial cells migrate to the desired location of the body. The endothelial cells elongate and align to create a solid sprout, while the lumen of the vessel is formed by a curvature in each endothelial cell [11]. The endothelial cells continue to proliferate, increasing the length of the sprout, until finally two hollow sprouts will join at their tips to create a loop and allow blood to flow. Pericytes then line the base of the loop and new sprouts can grow from its apex [12]. The exact process of these last steps of angiogenesis is not entirely understood, but the process is believed to be guided by specialized cells at the front of the sprout called “tip cell”.
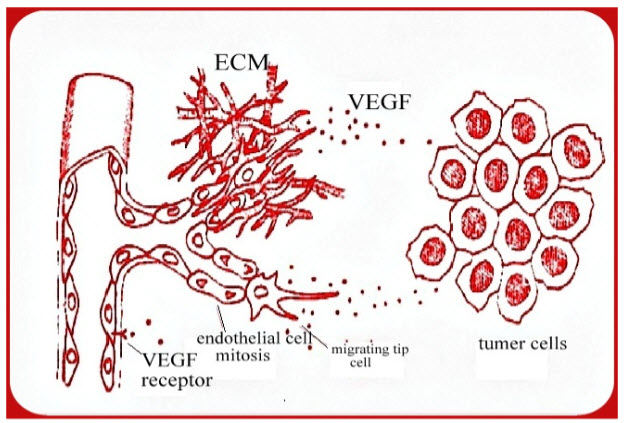
Figure 1: Mechanism of angiogenesis[13]
1.3 BODY CONTROL MECHANISM OF ANGIOGENESIS
Angiogenesis is an important natural process occurring in both the body of healthy and diseased individuals. Angiogenesis occurs in the healthy body for healing wounds and for restoring blood flow to tissues after injury. In females angiogenesis also occurs during the monthly reproductive cycle and during pregnancy. The healthy body controls angiogenesis through a series of "on" and "off" switches: The main "on" switches are known as angiogenesis stimulating growth factors and the "off switches" are known as angiogenesis inhibitors.When angiogenic growth factors are produced in excess of angiogenesis inhibitors the balance is tipped in favours of blood vesselgrowth. When inhibitors are present in excess of stimulators angiogenesis is stopped. The normal healthy body maintains a perfect balance of angiogenesis modulators. In general angiogenesis is "turned off" by the production of more inhibitors than stimulators. [14]
1.4 Table 1: HISTORICAL HIGHLIGHTS IN THE FIELD OF ANGIOGENESIS
|
Sr.No. |
Year |
Description |
|
1 |
1787 |
British surgeon Dr. John Hunter first called the term "angiogenesis" to describe blood vessels growing in the (Deer) Reindeer antler. |
|
2 |
1935 |
Boston pathologist Dr. Arthur Tremain Hertig describes angiogenesis in the placenta of pregnant monkeys. |
|
3 |
1971 |
Surgeon Judah Folkman supposes that tumour growth is dependent upon angiogenesis. |
|
4 |
1975 |
The first angiogenesis inhibitor is discovered in cartilage by Dr. Henry Brem and Dr. Judah Folkman |
|
5 |
1989 |
Vascular endothelial growth factor is discovered Dr. Napoleone Ferrara. |
|
6 |
1992 |
The first clinical trial of an anti angiogenic drug (TNP-470) begins in cancer patients. |
|
7 |
1997 |
The first angiogenesis-stimulating drug (Becaplermin, Regranex) is FDA-approved for treatment of diabetic foot ulcers. |
|
8 |
1998 |
The first angiogenesis-stimulating laser is FDA-approved for the treatment of severe, end stage coronary disease. |
|
9 |
1999 |
The first vascular targeting therapy is FDA-approved for treatment of age-related macular degeneration. |
|
10 |
2004 |
Pegaptanib becomes the first anti-VEGF drug to be FDA approved for the treatment of age-related macular degeneration. |
|
11 |
2005 |
Sorafenib (Nexavar) is a multi-tyrosine kinase inhibitor that demonstrates significantly longer progression-free survival vs. placebo in patients with advanced renal cancer in a randomized phase 3 trial. |
1.5 ANGIOGENESIS AND METASTASIS
Escape of the tumor cell from the confines of the primary tumor to distant body parts is the pre-requisite for hematogenous metastasis. This escape route is provided by the tumor vasculature. Thus, it was envisioned that inhibition of angiogenesis will also lead to inhibition of metastasis. This phenomenon was demonstrated by very elegant mouse model studies using angiostatin [20-21]. Angiostatin was also demonstrated to be secreted by some primary tumors leading to restricted growth of the metastasis leading to “dormancy” of the metastasis. Mice deficient in angiogenesis (Id1 & Id3 deficient) showed significantly less tumor take rates [22]. Independent studies showed absence of metastasis in angiogenesis deficient mice [23-24].Defective angiogenesis was attributed to impaired VEGF-dependent recruitment of precursor endothelial cells from the bone marrow to the newly developing tumor vasculature [25].
1.6 LYMPHANGIOGENESIS
Metastasis of malignant tumors to regional lymph nodes is one of the early signs of cancer spread in patients, and it occurs at least as frequently as hematogenous metastasis [26]Particularly, in cancers, such as breast cancer, lymphatic metastasis is a predominant route for tumor spread. The contribution of lymphatic system to the tumor growth is an area that is relatively less studied. However, lymphatic vessels are speculated to contribute to tumor growth and metastasis in a variety of ways. The VEGF, FGF2 and PDGF produced by vascular endothelial cells are proposed to be involved in the activation of lymphatic endothelial cells, which in turn produce matrix metalloproteases and urokinase plasminogen activator (uPA) that can promote malignant tumor growth. Thus, there exists a synergistic crosstalk between the tumor and the lymphatic vessels and blood vessels.
1.7 CAUSE AND CONCEQUNCESES OF ANGIOGENESIS
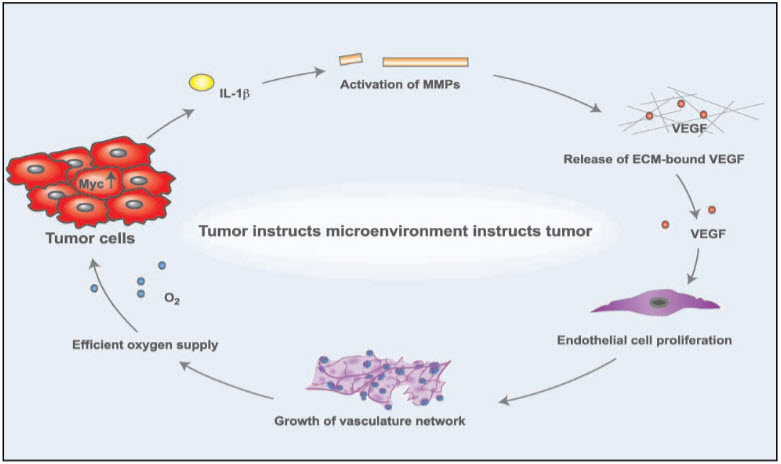
Figure 2: Cause of angiogenesis [27]
1.8 MEDICAL BACKGROUND
A growing tumor, after it reaches just a few millimeters in diameter, no longer can rely on blood vessels. Of the host for its supply of nutrients, but it needs to develop its own vessels and.capillaries for blood supply. In this process, called angiogenesis, there is a reciprocal.signaling between endothelial cells and tumor cells. Tumor cells produce vascular endothelial growth factor (VEGF) to stimulate endothelial cell growth; endothelial cells in turn provide the lining for the newly forming blood vessels that supply nutrients to the tumor and thus sustain tumor growth. But endothelial cells also have receptors which make them sensitive to inhibitors of inducers of angiogenesis like, for example, Endostatin, and pharmacologic therapies typically target the growth factor VEGF trying to impede the development of new blood vessels and capillaries. Overall, angiogenesis can be viewed as a complex balance of stimulatory and inhibitory mechanisms regulated through micro environmental factors [28].
1.8 REGULATORS OF TUMOR ANGIOGENESIS
Angiogenesis is a complex and intricately regulated process. Like all other regulated biological phenomena, angiogenesis has activators or pro-angiogenic factors and inhibitors or anti-angiogenic factors [29].
1.9 ACTIVATORS
Tumor cells activate signaling pathways that promote uncontrolled proliferation and survival. These include the PI3K/AKT/mTOR pathway, Hedgehog pathway and, Wnt pathway [30-31] that produce pro-angiogenic signaling intermediates [32-33] Among the several reported activators of angiogenesis present in cells two proteins appear to be the most important for sustaining tumor growth: vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). VEGF and bFGF are secreted by the tumor into the surrounding tissue. They bind to their cognate receptors on endothelial cells. This activates a signaling cascade that transmits a nuclear signal prompting target genes to activate endothelial cell growth. Activated endothelial cells also produce matrix metalloproteinase (MMPs). These MMPs break down the extracellular matrix and allow the migration of endothelial cells. The division and migration of the endothelial cells leads to formation of new blood vessels [34-35].
1.10 INHIBITORS
If angiogenesis is so critical for the tumor growth, then agents that inhibit angiogenesis would have great therapeutic value. With the discovery of Endostatin, the concept of anti-angiogenic therapy was launched and popularized by Dr. Folkman. Angiogenesis inhibitors have been discovered from a variety of sources. Some are naturally present in the human body e.g. specific fragments of structural proteins such as collagen or plasminogen (angiostatin, Endostatin, tumstatin. Others are natural products in green tea, soy beans, fungi, mushrooms, tree bark, shark tissues, snake venom etc. A plethora of synthetic compounds are also characterized to have anti-angiogenic properties [36]
1.11 ANTIANGIOGENIC THEREPY
Angiogenesis is the process of making new blood vessels. The term comes from 2 GreekWords: “angio” meaning blood vessel, and genesis, meaning beginning. Anti-angiogenesis is a form of targeted therapy that uses drugs or other substances to stop tumors from making new blood vessels. Without a blood supply, tumors can't grow.Anti-angiogenic therapy is an anti-cancer strategy that targets the new vessels that grow to provide oxygen and nutrients to actively proliferating tumor cells. Most of the current anti-cancer reagents used in the clinical setting indiscriminately target all rapidly dividing cells, resulting in severe adverse effects such as immunosuppression, intestinal problems and hair loss[37-38]Antiangiogenesis therapy is one of two types of drugs in a new class of medicines that restores health by controlling blood vessel growth. The other medication is called pro-angiogenic therapy[39].
1.12 PURPOSE
Antiangiogenic therapy inhibits the growth of new blood vessels. Because new blood vessel growth plays a critical role in many disease conditions, including disorders that cause blindness, arthritis, and cancer, angiogenesis inhibition is a "common denominator" approach to treating these diseases. Antiangiogenic drugs exert their beneficial effects in a number of ways: by disabling the agents that activate and promote cell growth, or by directly blocking the growing blood vessel cells [40].
1.13 CLASSIFICATION OF ANTIANGIOGENIC AGENTS
I. Drug that inhibit growth of endothelial cell: - Endostatin, Combrestatins, Thalidomide, Itraconazole, Silibinin.
II. Drugs that block Angiogenesis signaling: - Bevacizumab, Interferon α, Ranibizumab, Pegaptanib, Vetroprofin.
III. Monoclonal Antibodies:-Abciximab, Adalimumab, Alemtuzumab, Basiliximab, Belimumab, Bevacizumab, BrentuximabVedotin, Canakinumab, Cetuximab, Daclizumab, Denosumab, Eculizumab, Efalizumab, Gemtuzumab, Golimumab, Infliximab, Natalizumab, Omalizumab, Palivizumab, Panitumum, Ranibizumab, Rituximab, Tositumomab, Trastuzumab.
IV. Small Molecule Tyrosine kinase Inhibitors:- Erolatanib, Pazopanib, Sorafenib, Sunitinib Lapatinib, Dasatanib, Gefitinib, Canertinib, Vatalanib, Imatinib.
V. mTOR Inhibitors: - Deforolimus, Everolimus, Rapamycin, Temsirolimus.
VI. Metronomic Therapy:-Trofosamide, Cyclophosphamide, Capecitabine, Fluvastatin, Thalidomide, Vincristine, Docetaxel.
VII. Drugs that block extracellular matrix breakdown: -Batimastat (BB-94), Ro 32-3555, BB-2516(Marimastat), AG-3340 (Prinomastat), Actinonin
VIII.Miscellaneous:- Bortizomib, Doxorubicin, Gemcitabine, Dexamethasone, Fludarabine, Carboplatin, Topotecan
Table 2: Drug that inhibits growth of endothelial cell
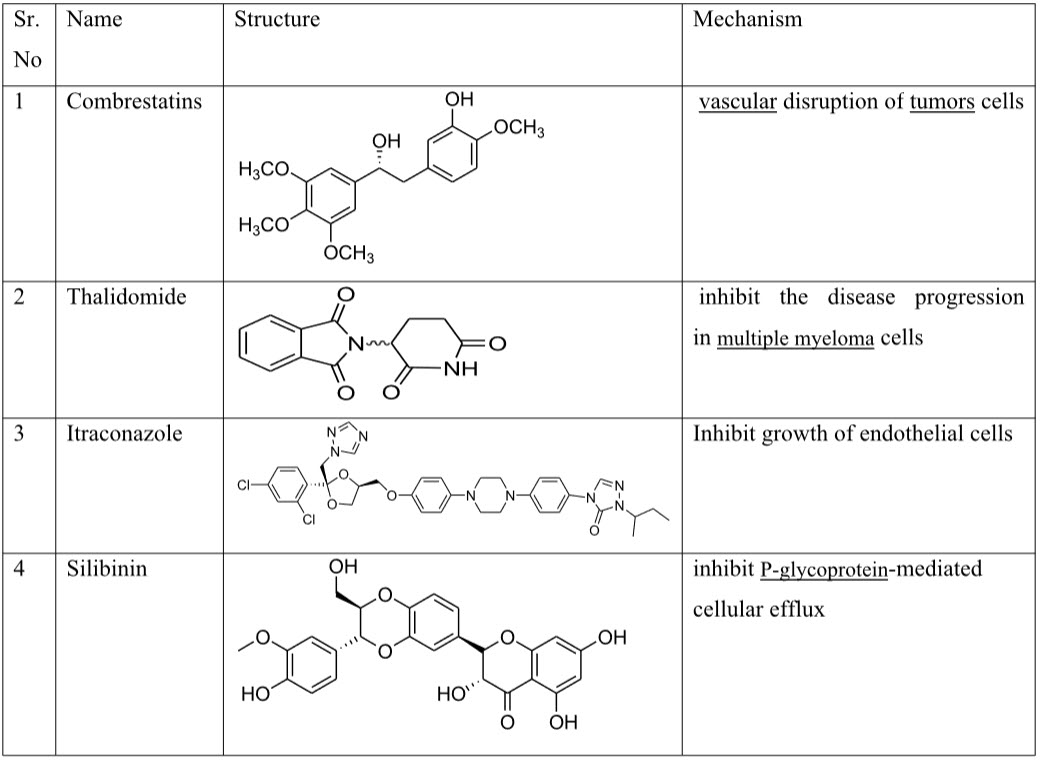
Table 3: Drugs that block Angiogenesis signaling
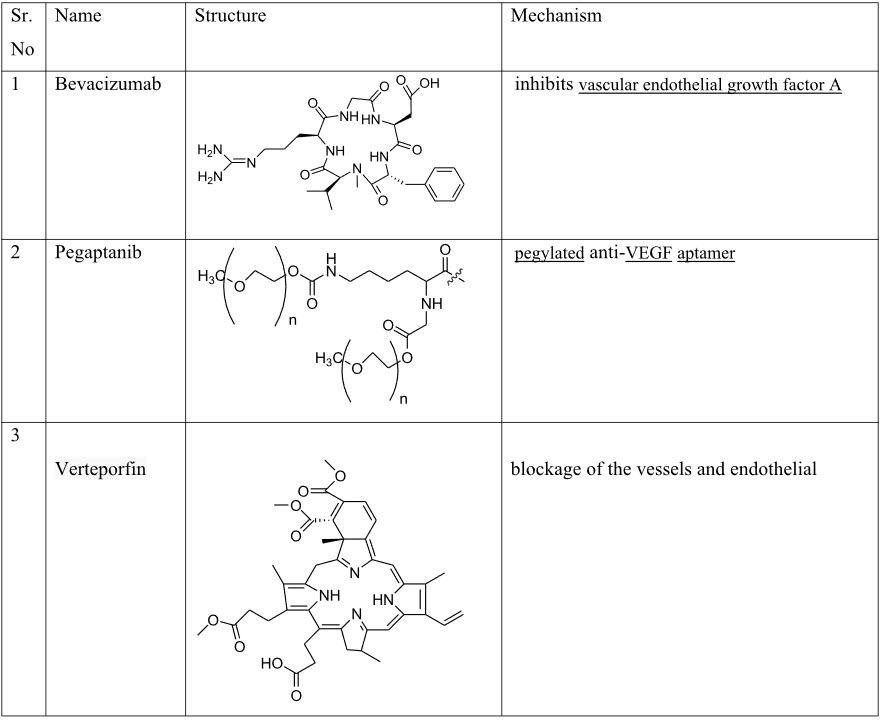
Table 4: Monoclonal Antibodies
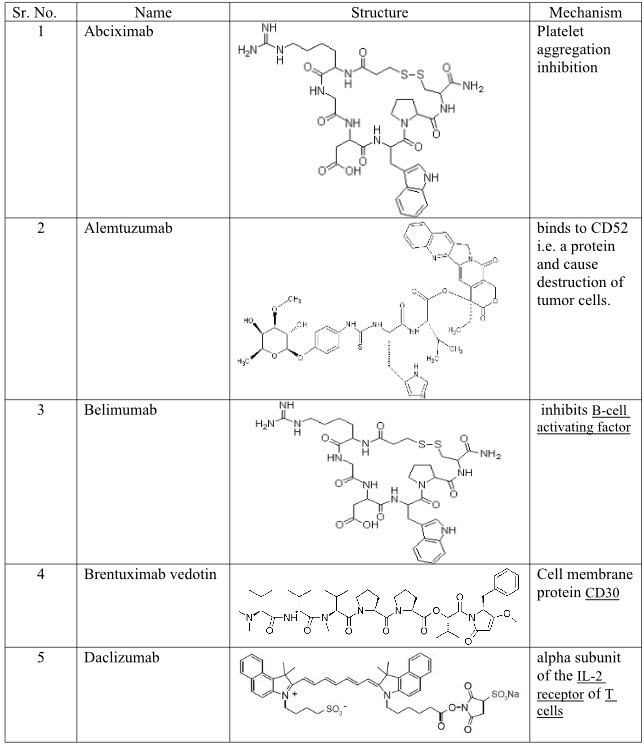
Table 5: Tyrosine kinase inhibitors
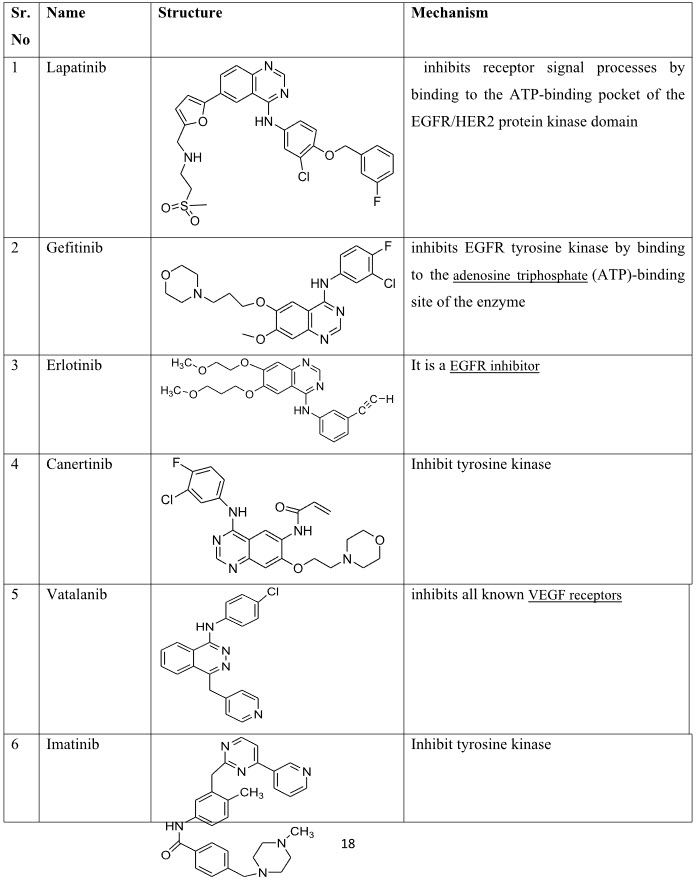
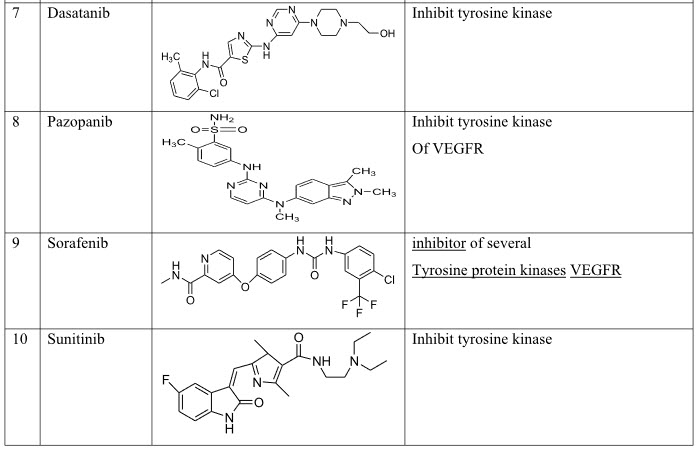
Table 6:mTOR Inhibitors
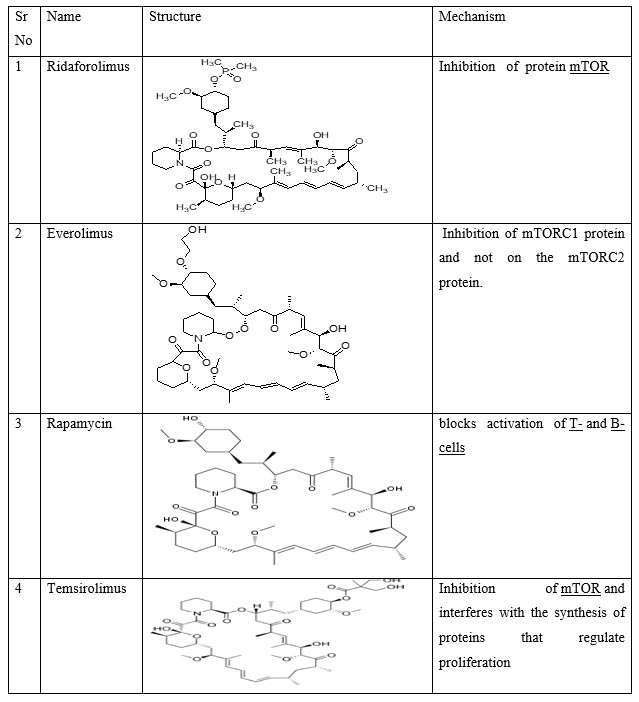
Table 7: Metronomic Therapy
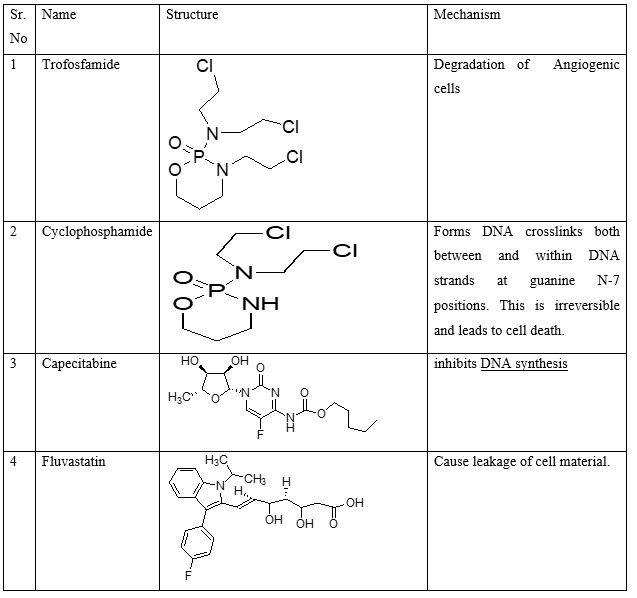
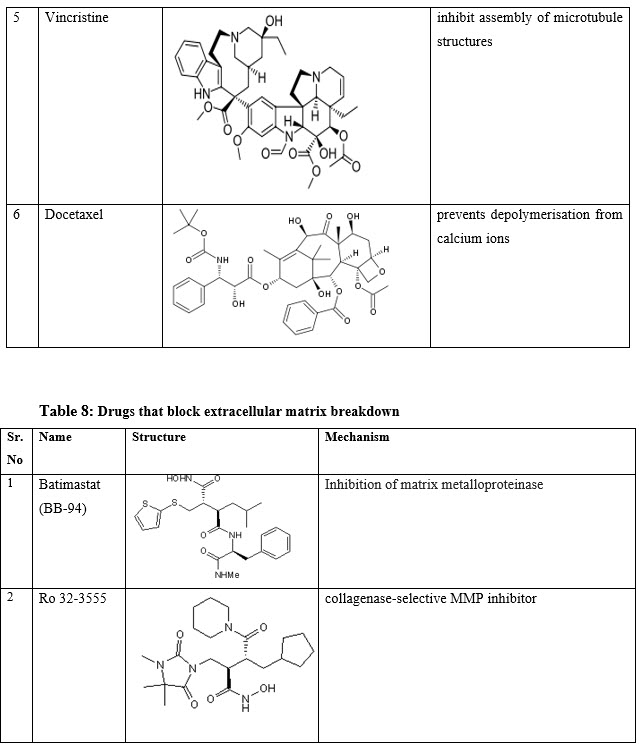
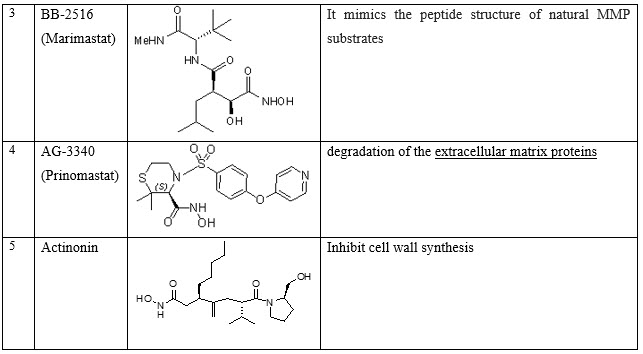
Table 9: Miscellaneous
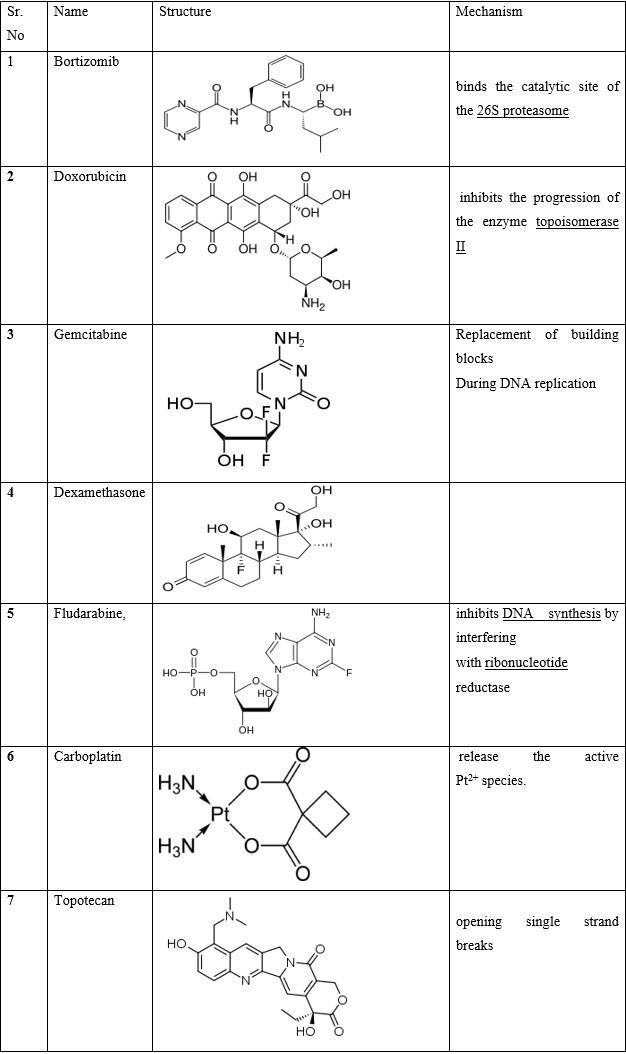
1.13 SIDE EFFECTS
Thrombosis
Most anti-angiogenesis drugs have been shown to raise the risk of internal bleeding or ofdeveloping a hole in the digestive tract. In rare cases, this has been serious or even fatal. In people with a history of bleeding problems, with certain types of cancers, or with cancers in certain locations, the risks of using these drugs might outweigh the benefits.
Hypertention
It's not clear why, but some of these drugs raise blood pressure. This problem is rarely serious and it seems to respond well to blood pressure medicines. Some people who have a history of high blood pressure, heart disease, or stroke may need to be watched closely or may not be able to take these drugs.
Surgery risks
Because they may affect wound healing, anti-angiogenesis drugs may need to be stopped before surgery or not started until a few weeks after surgery. This is to make sure blood vessels that are cut are able to repair themselves.
Pregnancy risks
These drugs might also affect a developing fetus. They will probably not be used forwomen who are pregnant or might become pregnant. [41]
Hypothyroidism and fatigue
The thyroid gland has many capillaries and Antiangiogenic. TKIs can affect thyroid homeostasis. In up to 36% of patients, an increase in thyroid-stimulating hormone and a decrease in the levels of the circulating thyroid hormones, indicative of hypothyroidism, have been observed after treatment with TKIs [42]. Disturbance in thyroid function might result in fatigue [43].
Skin toxicity
The inhibition of angiogenesis can also cause severe skin toxicities. Growth factor signaling pathways are involved in the homeostasis of the skin. For example, hair depigmentation,
hair loss and acral erythema are common during treatment with sunitinib or sorafenib [44]. The observation that endothelial cells are affected in the skin suggests that these agents have a direct consequence on the biological activity in skin toxicity. This toxicity might be relative to the interaction between stromal and endothelial cells.
Proteinuria and edema
Proteinuria, the presence of an excess of proteins in the urine, may be an indicator of renal damage. Renal function is partly regulated by VEGF [45], and the inhibition of VEGF causes mild proteinuria. Recently, Eremina et al.have found that local reduction of VEGF within the kidney is sufficient to trigger the pathogenesis of thrombotic microangiopathy and glomerular injury in patients who have been treated with bevacizumab and this is probably due to direct targeting of VEGF by anti-angiogenic therapy [46]. Because proteins are normally reabsorbed from urine, proteinuria is due to a decreased reabsorption or increased filtration. Moreover, when large amounts of proteins are lost in the urine, the balance of the osmotic pressure between the blood and the interstitium is disturbed. This will increase secretion of fluid into the interstitium or impair the removal of this fluid and cause edema.
Leukopenia, lymphopenia and immunomodulation
VEGFRs are expressed by almost all haematopoietic cells and endothelial cell precursors [47-48]. Therefore, it is possible that inhibition of angiogenesis can cause leukopenia and lymphopenia, as well as thrombocytopenia. In addition, VEGF is known to inhibit the functional maturation of dendritic cells from progenitors [49]. Therefore, the inhibition of VEGF may have an effect on the immune systemand induce immunomodulation.
1.14 CURRENT AND FUTURE PERSPECTIVES
Discussions on Current and Future perspectives in the field of angiogenesis are listed below.
Oncology
In the field of oncology angiogenesis inhibitors are used to treat Cancer. In U.S. there are currently eight approved anti-cancer therapies with recognized anti angiogenic properties agents. These agents which interrupt critical cell signalling pathways involved in tumour angiogenesis and growth involve three categories:
1) monoclonal antibodies directed against specific proangiogenic growth factors and/or their receptors.
2) small molecule tyrosine kinase inhibitors (TKIs) of multiple pro-angiogenic growth factor receptors.
3) inhibitors of mTOR(Mammalian target of rapamycin). In addition, there are two other approved anti angiogenicagents may indirectly inhibit angiogenesis through mechanisms that are not completelyunderstood.
Angiogenesis inhibitors have also been discovered from natural sources, including tree bark, fungi, shark muscle and cartilage, sea coral, green tea and herbs (liquorices, ginseng, cumin, garlic). In total, more than 300 angiogenesis inhibitors have been discovered to date. Near about 184 million patients in World could benefit from some form of anti angiogenic therapy [50-51].
Dermatology
In the field of dermatology there are several anti angiogenic agents used for neoplasm of the skin.
Ophthalmology
In the field of ophthalmology angiogenesis in the eye underlies the major causes of blindness in both developed and developing nations particularly age related macular degeneration (AMD), proliferative diabetic retinopathy (PDR), diabetic macular edema (DME), neovascular glaucoma, corneal neovascularization and pterygium. Presently approved anti-angiogenic therapies for ophthalmic conditions are biologic agents that inhibit VEGF. There are currently two approved anti angiogenic therapies for ophthalmic diseases an anti-VEGF aptamer (pegaptanib,Macugen) and a Fab fragment of a monoclonal antibody directed against VEGF-A (ranibizumab,Lucentis). The first FDA-approved blood vessel therapy for eye disease is a type of photodynamic therapy called Visudyne.This has shown effectiveness, for treating macular degeneration [52-53]
Diabetalogy
In the field of diabetics the first angiogenesis stimulating medicine is a prescription gel called Regranex that became FDA-approved to heal diabetic foot ulcers in December 1997.
Cardiology
In the field of cardiology angiogenic agents are used as a therapeutic angiogenesis for tissue repair and regeneration. Therapeutic angiogenesis modalities represent a broad range of interventions that generate new blood vessel growth to promote neovascularization and tissue repair. Near about 314 million patients in world would benefit from some form of angiogenesis stimulating proangiogenic therapy [54-55]. Presently, there are three major indications for which angiogenic therapies are in clinical use: 1) chronic wounds (e.g. diabetic lower extremity ulcers, venous leg ulcerations, pressure ulcers and arterial ulcers); 2) peripheral arterial disease; and 3) ischemic heart disease. In such conditions, the therapeutic goal is to stimulate angiogenesis to improve perfusion,deliver survival factors to sites of tissue repair, mobilize regenerative stem cell populations andultimately restore form and function to the tissue; More than 2,000 patients with heart disease have received some form of experimental angiogenic therapy. But currently there are no FDA approved angiogenic drugs for the treatment of ischemic cardiovascular disease. The first FDA approved device is used to stimulate new blood vessels to grow in diseased hearts is a laser used in a technique called Direct Myocardial Revascularization (DMR) or called as trans myocardial revascularization (TMR). So it is necessary to determine the potent angiogenic growth factors as well as research findings from natural sources are to be encouraged to alternate the recombinant protein, monoclonal antibody, device and cell based therapies [56-57].
CONCLUSION:
While angiogenesis as a hallmark of tumor development and metastasis is now a validated target for cancer treatment, the overall benefits of anti-angiogenic drugs from the perspective of impacting survival have left much to desire, endorsing a need for developing more effective therapeutic regimens e.g., combining anti-angiogenic drugs with established chemotherapeutic drugs.
RFERENCES
[1]. Angiogenesis: Potential for pharmacologic intervention in the treatment of cancer, cardiovascular diseases and chronic inflammation. Griffioen, A. W. and Molema, G. 2000, Pharmacological Reviews, Vol. 52, pp. 237-68.
[2]. Angiogenesis in the early human chorion and in primary placenta of the macaque monkey. Hertig, A. T.1935, Contributions to Embryology, Vol. 25, pp. 37-81.
[3]. Angiogenesis in cancer, vascular, rheumatoid and other disease. Folkman, Judah.1995, Nature Medicine, pp. 27-30.
[4]. Patterns and emerging mechanisms of the angiogenic switch during tumorogenesis. Hanahan, D. and Folkman, J.1996, Cell, Vol. 86, pp. 353-64.
[5]. Endothelial cell-mediated coagulation, anti-coagulation and fibrinolysis. Verstraete, M.Stuttgert, New York: Schattauer, 1995, The Endothelial Cell in Health and Disease.
[6]. Mechanisms of angiogenesis. Risaue, W. 1997, Nature, Vol. 386, pp. 671-74.
[7]. Nitric oxide synthase lies downstream from vascular endothelial grwoth factor-induced but not basic fibroblast growth factor-induced angiognesis. Ziche, M., et al.1997, Journal of Clinical Investigation, Vol. 99, pp. 2625-2634.
[8]. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and would healing. Dvorak, H. F.1986, New England Journal of Medicine, Vol. 315, pp. 1650-1659.
[9]. Activation of vascular endothlial growth factor gene trascription by hypoxia-inducible factor Forsythe, J. A., et al.1996, Molecular Cell Biology, Vol. 16, pp. 4604-4613.
[10]. Second wave of angiogenesis during KDR/Flk-1 antibody therapy (Abstract). Hansen-Algenstaedt, N., et al.199, Proceedings of the American Association for Cancer Research, Vol. 40, p. 620.
[11]. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Ausprunk, D. H. and Folkman, J.1977, Microvascular Research, Vol. 14, pp. 53-65.
[12]. Angiogenic Factors. Folkman, Judah and Klagsbrun, M.1987, Science, Vol. 235, pp. 442-447.
[13]. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. Gerhardt, Holger, et al. 2003, Journal of Cell Biology, Vol. 161, pp. 1163-1177.
[14]. The Jackson Cancer Modeling Group. Quantitative Cancer Research.[Online] 2011. [Cited: 06 13, 2011.] http://www.math.lsa.umich.edu/~tjacks/research.html.
[15]. P. Hahnfeldt, D. Panigrahy, J. Folkman and L. Hlatky, Tumor development under angiogenicsignaling: a dynamical theory of tumor growth, treatment response, and post vasculardormancy, Cancer Research, 59, (1999), pp. 4770-4775
[16]. Li W, Talcott K, Zhai A, Kruger E, Li V. Adv Skin Wound Care, 2005. 18, 491-500.
[17]. Froman interview with Dr. Folkman, NOVA online hatt. “www.pbs.org/wgbh/nova/cancer/ folkman html.2/011
[18]. Folkman J, Klagsbrun M. Science. 1987, 235, 442-447.
[19]. Kerbel RS. N Engl J Med. 2008, 358, 2039-2049.
[20]. Li W. Acad Radiol. 2000, 7, 800-811.
[21]. White CW, Sondheimer HM, Crouch EC. N Engl J Med., 1992. 326, 1456-1463.
[22]. Ingber D, Fujita T, Kishimoto S. Nature. 1990, 348, 555-557.
[23]. Smiell, JM, Wieman TJ, Steed DL. Wound Repair Regen.,1999. 7, 335-346.
[24]. Folkman J, Braunwald E, Fauci AS, Kasper DL. Tumor angiogenesis, Harrision’sTexbookof Internal Medicine, 15th Edition, McGraw-Hill, New York, 2000; 132-152
[25]. Folkman J, Devita VT, Hellman S, Rosenberg SA. Antiangiogenesis agents, Cancer: Principles & Practice of Oncology, 6th Edition, Lippincott Williams & Wilkins, Philadelphia, 2001; 509-519.
[26]. Andreoli CM, Miller JW. Cum op in Opthalmol.,2007, 18(6), 502-508.
[27]. Li WW, Hutnik M, Gehr G. Br J Haematol. 2008, 7372, 1365-2141.
[28]. Andreoli CM, Miller JW. Cum op in Opthalmol.,2007, 18(6), 502-508.
[29]. Jager RD, Mieler WF, Miller JW. N Engl J Med. 2008, 358(24), 2606-2617.
[30]. Folkman J, Braunwald E, Fauci AS, Kasper DL. Tumor angiogenesis, Harrision’sTexbook of Internal Medicine, 15th Edition, McGraw-Hill, New York, 2000; 132-152
[31]. Li WW, Li VW, Casey R. Clinical trials of angiogenesis based therapies: overview and newguiding principles, Angiogenesis: Models, Modulators and Clinical Application, Maragoudakis M. Edition, Plenum Press, New York, 1998, 475-492.
[32]. Li W. Acad Radiol. 2000, 7, 800-811.
[33]. White CW, Sondheimer HM, Crouch EC. N Engl J Med., 1992. 326, 1456-1463.
[34]. Smiell, JM, Wieman TJ, Steed DL. Wound Repair Regen.,1999. 7, 335-346.
[35]. Li VW, Kung EF, Li WW. Molecular therapy for wounds: modalities for stimulating Angiogenesis and granulation, Manual of Wound Management, 5th Edition, McGraw Hill, 2004,
[36]. Shubik P. Vascularization of tumors: A review.Journalof CancerResearch & Clinical Oncology. 1982; 103:211– 26
[37]. Reinhold HS, van den Berg-Blok A. Factors influencing the neovascularization of experimental tumours. Biorheology. 1984;21:493–501.
[38]. Goh PP, Sze DM, Roufogalis BD. Molecular and cellular regulators of cancer angiogenesis. Curr Cancer Drug Targets. 2007;7:743–58.
[39]. Kos M, Dabrowski A. Tumour's angiogenesis--the function of vegf and bfgf in colorectal cancer. Ann UnivMariae Curie Sklodowska [Med] 2002;57:556–61.
[40]. Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: Its emergence, its characteristics, and its significance. DevDyn. 2004;231:474–88.
[41]. Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, ScigallaP, Raymond E. Safety, pharmacokinetic, and antitumor activityof SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. Journal of Clinical Oncology 24: 25-35, 2006.
[42]. Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, Wechsler J, Lhomme C, Escudier B, Boige V, Armand JP, Le Chevalier T. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 6: 491-500, 2005.
[43]. de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am SocNephrol 12: 993-1000, 2001.
[44]. Hara A, Wada T, Furuichi K, Sakai N, Kawachi H, Shimizu F, Shibuya M, Matsushima K, Yokoyama H, Egashira K, Kaneko S. Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritis. Kidney Int 69: 1986-1995, 2006.
[45]. Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem 278: 12605-12608, 2003.
[46]. Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129-1136, 2008.
[47]. Katoh O, Tauchi H, Kawaishi K, Kimura A, Satow Y. Expression of the vascular endothelial growth factor (VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory effect of VEGF on apoptotic cell death caused by ionizing radiation. Cancer Res 55: 5687-5692, 1995.
[48]. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34 (+) cells identifies a population of functional endothelial precursors. Blood 95: 952-958, 2000.
[49]. Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency.Immunol Res 23: 263-272, 2001.
[50]. Ma J, Waxman DJ. Dominant effect of antiangiogenesis in combination therapy involving cyclophosphamide and axitinib. Clin Cancer Res. 2009;15:578–88.
[51]. Blagosklonny MV. Antiangiogenic therapy and tumor progression.Cancer Cell. 2004;5:13
[52]. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncology. 2002;29:158
[53]. O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a lewis lung carcinoma. Cell. 1994;79:315–28.
[54]. de Candia P, Solit DB, Giri D, Brogi E, Siegel PM, Olshen AB, Muller WJ, Rosen N, Benezra R. Angiogenesis impairment in id-deficient mice cooperates with an hsp90 inhibitor to completely suppress her2/neu-dependent breast tumors. Proc Natl Acad Sci U S A. 2003;100:12337–42.
[55]. Fong S, Itahana Y, Sumida T, Singh J, Coppe JP, Liu Y, Richards PC, Bennington JL, Lee NM, Debs RJ, Desprez PY. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis.Proc Natl Acad Sci U S A. 2003;100:13543–8. [PMC free article] [PubMed]
[56]. Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K, Gerald WL, Brogi E, Benezra R, Massague J. Id genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci U S A. 2007;104:19506–11.
[57]. Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









