About Authors:
Nimisha Paharia, Ankit Mittal, Garvita Joshi
MAHAKAL INSTITUTE OF PHARMACEUTICAL STUDIES,
UJJAIN (M.P.)
ABSTRACT
The pitfalls of needle-based injections are well known. A series of discoveries led to the development of the hypodermic needle which underwent significant changes. The first air-powered needle-free injection systems were developed during the 1940s and 1950s. Needle free delivery is done conveniently both for solids and liquids. Needle-free injection systems are typically made up of three components including an Injection device, disposable needle free syringe and air cartridge. Various needle free injectors are available in the market like Biojector, vitajet, iject, cool.click etc. These formulations are designed for better acceptability and patient convenience. They offer less pain and no needle phobia. They are ideally suited to chronic injections of varying doses of insulin, proteins and monoclonal antibodies.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1178
INTRODUCTION
The demand for novel drug delivery technologies is ever increasing. These drug delivery technologies can be broadly classified into four principle routes like oral, transdermal, inhalation and parenteral. The main goal for the delivery of any drug therapy is oral administration with once or twice daily dosing. However, there are large numbers of therapies, particularly protein-based, gene-based; vaccine-based that cannot be delivered by this route for example insulin, growth hormones and other similar biologics.
Pulmonary delivery is another non-invasive alternative method that is suitable for small molecules and proteins. However, for drugs with very large molecular weights, such as monoclonal antibodies, penetration through the lung for systemic delivery may require some type of transport enhancement mechanism, of which there are several still at the primordial research stage. Therefore, most protein-based drugs are still being developed as injectables for initial market launch.
The pitfalls of needle-based injections are well known. Psychological resistances to self-injection or needle-phobia have been documented across large demographic groups, such as diabetics. The result of this phobia is that many outpatient injectables are dosed sub-optimally. Furthermore, awareness of serious problems has caused physicians and their patients to either delay therapy initiation or seek out less-invasive alternatives, even at some cost to clinical effectiveness.
For some, especially those suffering from chronic diseases requiring injectable products two or three times a day, this process is an ongoing reality of daily life for example diabetics-accepted, but always with the hope that something new will replace the ritual of needle insertion. To overcome the problems related to needle based injections, there is one technology that has received considerable attention during the past few years and that offers all of the sought after benefits is—Needle Free Injection Technology (NFIT).[1]
History
As long as drugs have been known to cure diseases, people have searched for better methods of delivering them. During the early nineteenth century researchers made a series of discoveries that eventually led to the development of the hypodermic needle by Alexander Wood in 1853. This device was used to give morphine to patients suffering from sleeping disorders. In subsequent years, the hypodermic needle underwent significant changes which made them more efficient to use, safer, and more reliable. However, needles still have significant drawbacks which prompted researchers to find needle-free alternatives.
The first air-powered needle-free injection systems were developed during the 1940s and 1950s. These devices were gun-shaped and used propellant gases to force fluid medicines through the skin. Over the years, the devices have been modified to improve the amount and types of medicines delivered, and the efficiency and the ease of use.
Raw Material
Since these devices directly contact the body, they must be made from materials that are pharmacologically inert. The materials also must be able to withstand high temperatures because they are heat-sterilized. Air forced injection systems are available in different shapes and sizes. The outer shell of the device is made from a high strength, lightweight thermoplastic such as polycarbonate. Polycarbonates are polymers produced synthetically through various chemical reactions. To make the polymer easier to mold, fillers are added. These fillers make plastics more durable, lightweight, and rigid. Colorants are also incorporated into the plastic to modify the appearance. Prior to manufacture, the plastics are typically supplied in pellet form with the colorants and fillers already incorporated. Air-forced systems typically use carbon dioxide or helium gas to propel the medicine into the body.
[adsense:468x15:2204050025]
The three different types of injections:
Intradermal
Intramuscular
Subcutaneous
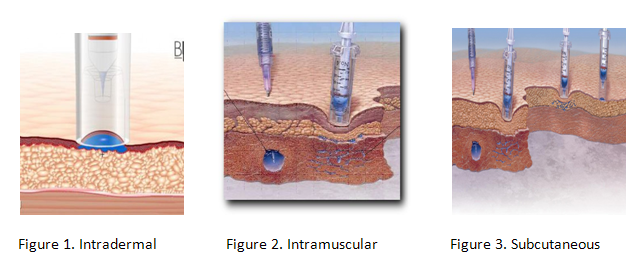
Certain types of medicines work better with needle-free injection systems than other. Insulin, which must be administered daily to diabetics, can be incorporated into an inhaler system. Lidocaine hydrochloride, a local anesthetic is suitable to be delivered needle free. Other medicines suitable for needle free systems include Fentanyl (an opioid analgesic), Heparin (an anticoagulant) and a variety of vaccines. Various adjunct ingredients included in these medicines include cyclodextrins, lactose, liposomes, amino acids and water.[2]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Design
The air-forced needle-free injection systems are typically made up of three components including an:
· Injection device
· Disposable needle free syringe
· Air cartridge
The injection device is made of a durable plastic. It is designed to be easy to hold for self-administration of medicine. The needle-free syringe is also plastic. It is sterilized and is the only piece of the device that must touch the skin. The syringe is made to be disposed after every use. For portable units, pressurized metal air cartridges are included. Less mobile devices have air hook-ups that attach to larger containers of compressed air. Some air-forced systems use a reusable spring to generate the pushing force instead of pressurized air cartridges.[3]
The Manufacturing Process
There are numerous methods of producing each needle-free injection system. The following process focuses on the production of an air-forced system. These systems are made through a step by step procedure which involves molding the pieces, assembling them, and decorating and labeling the final product. The individual pieces are typically produced off-site and assembled by the needle free injection system manufacturer. All of the manufacturing is done under sterile conditions to prevent the spread of disease.
Making the pieces
- The first step requires the production of the component plastic pieces from plastic pellets. This is done by a process called injection molding. Pellets of plastic are put into a large holding bin on an injection molding machine. They are heated to make them flowable.
- The material is then passed through a hydraulically controlled screw. As the screw rotates, the plastic is directed through a nozzle which then injects it into a mold. The mold is made up of two metal halves that form the shape of the part when brought together. When the plastic is in the mold, it is held under pressure for a specified amount of time and then allowed to cool. As it cools, the plastic inside hardens.
- The mold pieces are separated and the plastic part falls out onto a conveyor. The mold then closes again and the process is repeated. After the plastic parts are ejected from the mold, they are manually inspected to ensure that no significantly damaged parts are used.
Assembling and labeling
- The parts are next transported to an assembly line. In this production phase various events occur. Machines apply markings that show dose levels and force measurements. These machines are specially calibrated so each printing is made precisely. Depending on the complexity of the device, human workers or machines may assemble the devices. This involves inserting the various pieces into the main housing and attaching any buttons.[4]
Packaging
- After the assembly step, the injection devices are put into packaging. They are first wrapped in sterile films and then put into cardboard or plastic boxes. Each part is packaged so movement is minimal to prevent damage. For consumer products, an instruction manual is included along with safety information. These boxes are then stacked on pallets and shipped via truck to distributors.
Quality Control
Quality control checks are done throughout the manufacturing process. Line inspectors check the plastic components to assure they conform to predetermined specifications. Visual inspections are the first test method, but measuring equipment is also used to check the dimensions including size and thickness. Instruments that can be used include laser micrometers, calipers and microscopes. Inspectors also check to make sure the printing and labeling is correct and that all the parts are included in the final packages.
Since these devices can have various safety issues, their production is strictly controlled by the Food and Drug Administration (FDA). Each manufacturer must conform to various production standards and specifications. Announced and unannounced inspections may occur to ensure that these companies are following good manufacturing practices. For this reason detailed records must be kept related to production and design.[5]
NEEDLE FREE DELIVERY OF SOLIDS
As well as the obvious advantages for liquid formulations, such is needle phobia etc. described above, delivering the drug or vaccine in a solid dosage form has the additional advantages that the therapeutic agent will typically be more stable and may not require cold chain storage. In addition, a solid formulation presents the opportunity to combine fast-acting and delayed-release forms such as for vaccines so that the ‘prime’ and ‘boosts’ shots can be given together in a single administration.
NEEDLE FREE DELIVERY OF LIQUIDS
Needle-free injectors have the obvious advantages that they avoid issues relating to needle phobia, needle disposal and the potential for cross contamination of blood-borne diseases. Probably the most well-known needle-free technologies involve liquid jet injection. Liquid jet injector technology was first developed many decades ago and yet it is still not widely used although there are products based on some of these technologies on the market. One of the main attributes of the liquid jet injectors is that these use the drug in a liquid form which therefore does not typically require re-formulation from standard needle and syringe formats. The jet injectors have been developed as both single-use devices and multi-use systems. All require a power source that provides a very high peak pressure behind the liquid in order that it can ‘drill’ a hole in the skin, without the use of a needle, followed by a reduced pressure profile to force the rest of the liquid into the skin. This requires careful control over the power source to ensure accurate and reliable delivery of the drug to different skin locations on the same person. A variety of power sources has been developed for these liquid jet injectors, including:
· Springs
· Compressed gas
· Controlled chemical reactions[6]
Needle Free Injectors
Biojector 2000
The Biojector 2000(Figure 4) is a durable, professional-grade injection system designed for healthcare providers. The Biojector 2000 is the only needle-free system in the world cleared by the FDA to deliver intramuscular injections. The system can also deliver subcutaneous injections, and is being used for intradermal injections in clinical trials.
The Biojector 2000 uses sterile, single-use syringes for individual injections, which prevent the cross-contamination that has been reported with fixed-nozzle jet injection systems.
More than 10 million injections have been administered successfully using the Biojector 2000, with no reports of major complications. Because there is no needle, the Biojector provides healthcare workers with an unparalleled level of protection against accidental needle stick injuries. In high-risk situations, such as delivering injections to patients known to be infected with HIV or hepatitis, the Biojector is an ideal injection system.
Vitajet
The Vitajet 3(Figure 5) is an easy-to-use, economical needle-free injection system for delivering insulin. The system requires no maintenance or re-assembly. With disposable nozzles that are replaced once-a-week, the Vitajet 3 offers the quality of a reusable medical product, with the convenience and safety of a sterile disposable.  The exclusive, easy-to-read Crystal Check disposable transparent nozzle allows inspecting the dosage prior to injection and visually confirming loading and full discharge of your insulin after each use.
The exclusive, easy-to-read Crystal Check disposable transparent nozzle allows inspecting the dosage prior to injection and visually confirming loading and full discharge of your insulin after each use.
The Vitajet 3 received the FDA marketing clearance for delivering subcutaneous injections of insulin in 1996. Since then, the system has been used to deliver hundreds of thousands of injections, safely, economically, and without the use of a needle.
Cool.click
Bioject developed the cool.click (Figure 6) needle-free injection system for delivering Saizen recombinant human growth hormone. In some children, naturally occurring growth hormone is absent or is produced in inadequate amounts. In these cases, Saizen or growth hormone replacement must be injected to maintain normal growth.
In these cases, Saizen or growth hormone replacement must be injected to maintain normal growth.
Cool.click is a customized version of Bioject’s Vitajet 3 needle-free injection system. The system includes customized dosage features to accurately deliver variable doses of Saizen and was designed with bright colors to make the injector attractive and non-threatening to children. The cool.click received FDA market clearance for delivering subcutaneous injections of Saizen in June, 2000.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
SeroJet
The SeroJet(Figure 7) is a needle-free injection system for delivering Serostim recombinant human growth hormone for treatment of HIV-associated wasting in adults.  HIV-associated wasting is a metabolic condition in which people infected with HIV lose body weight. If not treated, this could result in increased morbidity and mortality.
HIV-associated wasting is a metabolic condition in which people infected with HIV lose body weight. If not treated, this could result in increased morbidity and mortality.
Serono developed Serostim to treat this condition by utilizing the natural properties of growth hormone in increasing lean body mass. SeroJet is a customized version of Bioject’s Vitajet needle-free injection system. The system includes customized dosage features to accurately deliver variable doses of Serostim. The SeroJet received FDA market clearance for delivering subcutaneous injections of Serostim in March 2001.
Iject
Bioject has developed a second-generation gas-powered injector known as the Iject (Figure 8), which is based on the design and performance of the B2000 and is intended to serve as a single-use pre-filled device. The pressure profile of the Iject has been documented by in vitro testing to be virtually the same as that of the B2000, and injection performance of the two devices is therefore predicted to be equivalent.
 The Iject is a pre-filled single-use disposable injection device configured to administer 0.5 to 1.00 ml subcutaneous or intramuscular injections. The device is distributed “ready to use.” Thus, it requires no additional parts or modifications for function.
The Iject is a pre-filled single-use disposable injection device configured to administer 0.5 to 1.00 ml subcutaneous or intramuscular injections. The device is distributed “ready to use.” Thus, it requires no additional parts or modifications for function.
The device is primed by rotating the trigger sleeve 180 degrees, and an injection is administered by advancing the trigger sleeve while the nozzle is held against the injection site. The Iject needle-free injection system is an investigational device, subject to the US Food and Drug Administration clearance for commercial distribution.
Non-invasive DDS: Untapped potential
Aradigm Corporation has recently acquired the Intraject technology, initially developed in the UK by Weston Medical. It is the only pre-filled and disposable needle-free device in late-stage development, with commercial scale-up in process. Aradigm’s Intraject collaborators include Roche for the delivery of pegylated interferon alpha (Pegasys) and GlaxoSmithKline for Imitrex.
The Intraject device is about the size of a fountain pen. The drug capsule is glass, a material that has demonstrated excellent stability profiles for liquid protein formulations. The energy to drive the actuator forward to deliver the 0.5-ml formulation is provided by compressed nitrogen. The delivery process is completed in less than 60 milliseconds with less bruising and discomfort than may be encountered with syringes, pens or other devices.
Biovalve’s Mini-Ject technology
The Mini-Ject represents the next generation in needle-free injection systems by combining the features of accuracy reliability, a variety of pre-filled options, comfortable administration, and full disposability, all within a patient friendly easy-to-use design. The Mini-Ject can deliver a wide range of drugs, ranging from small molecules to large proteins, fragile antibodies, and vaccines. Delivery can be targeted to intradermal, subcutaneous or intramuscular depending on the clinical need. No other single-use needle-free delivery technology provides the same level of performance as the Mini-Ject technology with the ability to target specific tissue layers over such a broad range of drug volumes (0.1 mL to 1.3 mL) and viscosities.[7]
SOME NEEDLE FREE INJECTORS AVAILABLE IN THE MARKET
|
Technology/product name |
Company name |
Description |
|
Implaject |
Caretek Medical |
Simple, hand-held needle free injection device. Can be configured to be reusable with disposable cartridges.[8] |
|
PowderJect |
PowderMed |
It painlessly delivers DNA vaccines to the skin in a dry formulation.[9] |
|
Zoma-jet 2 Vision |
Antares Pharma |
Customized version ofMedi-jector vision licensed to Ferring for administration of their human growth hormone, Zomacton for distribution in Europe.[10] |
|
Valeo (MJ8) |
Antares Pharma |
Next generation pen-style, spring-powered device. Designed for use with drugs in cartridge containers, rather than vials.[11] |
|
Injex 30 |
Injex (HNS International) |
Spring-powered hand-held device with disposable ampoules that delivers 0.05-0.3 ml. Focused on insulin delivery.[12] |
|
Intraject |
Weston
|
Applicable |
|
Medi-Jector vision
|
Antares Pharma, lnc
|
It |
|
Penjet |
Penjet Corporation |
A pre |
|
Med-
|
Evans |
Needle |
|
Crossject |
Crossject |
Prefilled, single use disposable NFI. Uses chemical reaction to generate propellant at the time of administration.[17] |
Advantages
Needle Phobia”, “Needle-stick injuries and contamination”, "Patient Care”, "Self administered injectables” and “Emergency situations” are important public health issues where needle free devices can bring significant improvements.
· In improving patients’ health: Better comfort of administration, better acceptance of heavy chronic treatments and consequently better treatments compliance
· In avoiding mistakes for patients and healthcare workers: Drug, dosage, depth of injection
· In allowing self-administration
o Increases patients independence and reduces the risk of missed or incorrect injections or drug discontinuation due to unreliable injection assistance
o Reduces health-care cost
· In eliminating needle-stick injuries and associated contamination and the consequent dramatic social, psychological and economic consequences
· In allowing -in emergency situations- quick, efficient and non-traumatizing injections.
· In avoiding the risks and the costs associated to the elimination of contaminated sharps.
· In offering the pharmaceutical and biotech companies a unique opportunity of differentiation in a context of “life cycle management” of their products.
· Patients can easily administer their treatment chronically and from home or at work if needed.
· The medical staff has the guaranty of safe and quick delivery of the right treatment at the right dose in the right place.
· Pharmaceutical companies offer patients and practitioners a unique and convenient way of administering their products and a way to differentiate.
· Avoiding needle stick injury.
· Reduced sharps disposal: Disposal of sharp medical waste requires costly sharps disposal services. PharmaJet's needle-free syringe can be disposed in the same way a used Band-aid is disposed - thus making it simple and inexpensive.[18],[19]
CONCLUSION
The principles which were the basis of the use of the drugs to penetrate the skin, whether the drug is in solid, and liquid or in powder form have been established for many years. Needle free injection technology offers effective injectors for a wide range of drugs & bioequivalent to needles and syringes. Needle free devices have demonstrated consistent delivery to the epidermis, the dermis, the subcutaneous and the intramuscular space. They offer less pain, avoid needle stick injuries and contamination, allows self administration and results in no needle phobia and are thus strongly preferred by the patients. Some of them are ideally suited to chronic injections of varying doses of insulin, proteins and monoclonal antibodies.
REFRENCES
1. Vivek Ranjan Sinha, Aman Trehan, Pramil Tiwari:”Needle Free Injection Technology”:Express Healthcare Management (1st-15th July 2005) expresshealthcaremgmt.com/20050715/pharma01.shtml
2. Henry, C. "Special Delivery." Chemical & Engineering News (September 18, 2000): 49-65.
3. Potera, C. "Making Needles Needless." Technology Review (September/October 1998): 67-70.
4. Potera, C. "No-Needle Vaccine Techniques." Genetic Engineering News (August 1998): 19.
5. Seppa, N. "Edible Vaccine Spawns Antibodies to Virus." Science News (July 22, 2000): 54.
6. iptonline /articles/public/page78nonprint.pdf
7. Vivek Ranjan Sinha, Aman Trehan, Pramil Tiwari:”Needle Free Injection Technology”:Express Healthcare Management (1st-15th july 2005) expresshealthcaremgmt.com/20050715/pharma01.shtml
8.glidepharma.com/lead-drug-candidate-for-implaject.html
9. in-pharmatechnologist.com/Materials-Formulation/PowderJect-renewed-in-Chiron-spin-out
10. antarespharma.com/products/
11. wikinvest.com/stock/Antares_Pharma_(AIS)/Development_Efforts_Mj8_Valeo_Needle-free_Injection_Systems
12. injex.org/?q=node/6
13. iptonline.com/articles/public/IPTFIVE100NP.pdf
14. mediject.com/about/overview.htm-
15. penjet.com/pages/pr_degas.html
16. cdc.gov/nip/dev/N¬3draft0007
17. crossject.com/
18. American Nurses Association; "2008 Study of Nurses' Views on Workplace Safety and Needlestick Injuries"; Summer 2008
19. World Health Organization; "(GIVS) Global Immunization Vision and Strategy 2006-2015"; October 2005; page 44.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









