{ DOWNLOAD AS PDF }
ABOUT AUTHOR
Devender Sharma*
Hi – Tech college of pharmacy, Chandrapur, Maharashtra (India)
*sdevender350@gmail.com
ABSTRACT
Transdermal drug delivery carried out a promising carrier in the transport of drugs to get direct access across the skin deep into the systemic circulation. Transdermal drug delivery have a number of advantages including improved patient compliance, sustained release, avoidance of gastric irritation, as well as elimination of pre-systemic first-pass effect. It gives attraction to many researchers due to various biomedical advantages. Due to the limitation of oral drug delivery system and the pain related with the use of needles in case of injections, drug delivery research has tremendously oriented towards the transdermal route. Delivery of drugs via transdermal route has proved to be the convenient route for various clinical implications. The objective of the present review is to focus on newly innovations in transdermal drug delivery systems which can create a platform for the research and development of pharmaceutical drug dosage form for efficient transdermal delivery. In this review, we tell about different types of microneedles are described and their methods of fabrication. Microneedles can be fabricated in different forms like hollow, solid, and dissolving. There are also hydrogel-forming microneedles. In relation to hydrogel-forming microneedles, special attention, these are innovative microneedles which does not contain drugs but imbibe interstitial fluid to form continuous conduits between dermal microcirculation and an attached patch-type reservoir. Regulatory authorities approved several microneedles for clinical uses are also examined. The last part of this review discusses concerns and challenges regarding microneedles use.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2553
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 1 Received On: 25/09/2017; Accepted On: 26/09/2017; Published On: 01/01/2018 How to cite this article: Sharma D; Microneedles: an approach in transdermal drug delivery: a Review; PharmaTutor; 2018; 6(1); 7-15; http://dx.doi.org/10.29161/PT.v6.i1.2018.7 |
INTRODUCTION
Drug delivery via transdermal route across the skin provides the most convenient route for various clinical implications deep into the systemic circulation and it developed for controlled drug delivery. Transdermal drug delivery system represents a system which delivers the drug (therapeutically active amount of drug) effectively across the human skin. These are generally describes as the devices, which holds drug molecules of defined surface area that delivers the predetermined amount of drug to the intact skin surface at a predetermined rate. These systems have been designed to deliver the drug through the skin to the systemic circulation. It is defined as a self-contained, innovative delivery system which is considered to deliver the drug upon application into the skin, at controlled rate to the systemic circulation. (Dhamecha et al., 2009) Hypodermic needles generally used in clinical practice to deliver medications across the skin into the bloodstream. Injections with hypodermic needles are important from a clinical standpoint, but painful. They may induce hypersensitivity; bruising, discomfort and bleeding at the site of administration, and in some cases are associated with risks of contamination. (Nida et al., 2014) There are other concerns linked to their use including accidental needle stick injury and the necessity to train medical staff regarding the proper use of needles. (Rani et al., 2011) The difficulty in crossing the skin is caused by its anatomical peculiarities. (Arunachalam et al., 2010) The skin is the largest organ in the body. It is about 1.5 m2 in adults and provides protection for internal organs and also protects the human body against ingress of toxic chemicals and egress of water. Despite the large surface area of the skin, it is challenging for compounds including drugs and vaccines to cross the skin in therapeutically relevant amounts. (Ehdaie et al., 2011) The outer most layer of the skin i.e. stratum corneum plays role of major barrier in the skin. The stratum corneum is 10–15 μm thick with 15–20 corneocyte layers. It is made up of corneocytes embedded in an intercellular lipid matrix. In human stratum corneum, the main lipid classes are free fatty acids, ceramides and cholesterol which form two lamellar phases. These include the short periodicity phase and the long periodicity phase with repeat distances of approximately 6 and 13 nm, respectively. Below the stratum corneum is the viable epidermis, which is a cellular, avascular tissue measuring 50–100 μm thick. (Prausnitz et al., 2011) The viable epidermis consists mainly of keratinocytes and approximately 40% protein, 40% water and 15%–20% lipids. The undulating epidermal–dermal junction consists of papilla that project into the dermis. Cells in the basal layer of the epidermis form the most important structural and functional connection to the dermis below. (Lhernould et al., 2015) The stratum corneum and viable epidermis together form the full epidermis. There is a basement membrane at the base of the epidermis and the existence of tight junctions in the viable epidermis has been recently documented. Base membrane and tight junctions may both offer resistance to the transport of molecules across the epidermis. (Andrews et al., 2013) Below epidermis layer is the reticular dermis, made up of thick collagen bundles and coarse elastic fibers. The dermis contains blood vessels, lymphatics and nerves, as well as the various skin appendages. Below the reticular dermis lies the hypodermis (subcutaneous fat tissue), which may have a thickness of up to several millimeters. (Jepps et al., 2013).
Advantages of transdermal drug delivery
There are number of advantages related with the use of transdermal system for the effective delivery of drugs systemically and it includes improved patient compliance, avoids first pass hepatic metabolism in comparison to oral drug delivery systems. (Olatunji et al., 2013) This system reduces the adverse effects regards with the drug caused due to overdose and is a convenient route and comprises of simple dosing, especially in case of transdermal patches that require only once in a week application which helps in patient adherence to drug therapy. It avoids gastrointestinal irritation and avoids gastrointestinal absorption and enzymatic related deactivation, and reduces fluctuations in plasma drug profile. It enhances the bioavailability as well as high concentrations of drugs delivered via this route can be localized at the site of action, thereby reducing the systemic drug levels. (Cheung et al., 2014) Drug delivery through transdermal route is an attractive method to transport drug or biological compounds due its advantage in reducing the pain and inconvenient intravenous injections. (Kim et al., 2012)
Disadvantages of transdermal drug delivery system (Verbaan et al., 2008)
It possessed some limitation such as local irritation, erythema, itching, and local oedema may be produced by the drug or other excipients at the site of application especially in the patch formulation. Limited permeability across through the skin may limit the delivery of number of drugs. Various attempts have been made to overcome these limitations and make its conventional route. (Vinayakumar et al., 2014)
Microneedles
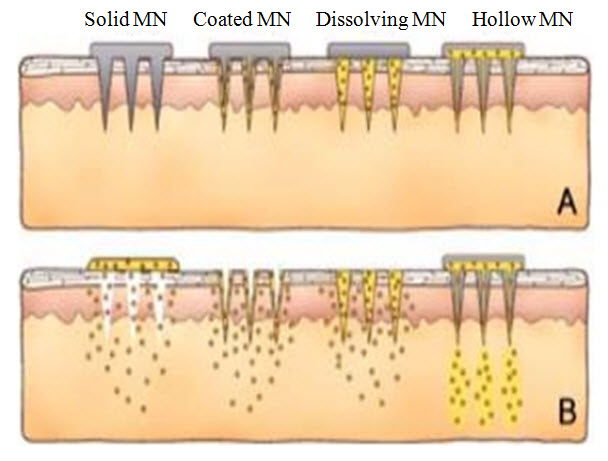
Microneedles are recently developed systems for drug delivery which is similar or likely to traditional needles but the difference is these are fabricated on the micron scale and the size ranges from 1-100 microns in length and 1 micron in diameter. These are defined as micro-scale needles, arranged on a transdermal patch. Microneedles are currently being utilized to enhance transdermal delivery of small and large molecules. (Gupta et al., 2011) Transdermal microneedles create micron sized pores in the skin to enhance delivery of the drug across the skin. When microneedles are fabricated in arrays on a backing that can be applied to the skin like a bandage, the device is called a microneedles patch. (Henry et al., 1998) Microneedles can be divided into four categories like hollow, solid, coated and polymer. Hollow microneedles are like regular hypodermic needles but shorter in length. A liquid formulation of the drug is infused through bores in the microneedles. (Wang et al., 2012)Solid microneedles are used to create holes in the skin. Subsequently a patch is then applied. Coated microneedles are microneedles coated with the drug while polymer microneedles are made by polymers that can be dissolving, non- dissolving or hydrogel-forming. (Sullivan et al., 2010)
Characteristics of microneedles
The characteristics of micro needles include ruggedness, microneedles developed must be capable of insertion deep into the skin without breaking. (Hong et al., 2013)They should be manufactured by taking optimum size and if they are too long, upper portion of micro needles may not have enough rigidity and could undergo breakage before penetration. Microneedles generally used in controlled drug released, they should deliver the controlled amount of drug at a definite and predetermined rate. The micro needles should be able to penetrate the drug to the required depth in the tissues of the body. (Chen et al., 2013) Generally, the dimensions of micro needles can vary depending on the types of microneedles. Typical microneedles geometries may ranges from 150-1500 microns in length, 50-250 microns in base width, and 1-25 microns in tip diameter. The tips of microneedles are of different shapes like triangular, rounded or arrow shaped. (Pearton et al., 2012)
Most of microneedles make by materials includes glass, silicone (of brittle nature), metals such as stainless steel, solid or coat of gold over nickel, palladium, cobalt and platinum and biodegradable polymers. (Khan et al., 2014)
Effective characteristics in case of ideal microneedles, designing of microneedles can be such so as to minimize the pain. Various studies revealed that specific micro needles of about a couple hundred microns length were reported to be painless. It was reported by various authors that l3-times increment in needle length (i.e., 500-1500 microns) increases the pain by 7 times (i.e., 5-35% caused by hypodermic needle). If the length remains constant, an increase in number of microneedles (i.e., 620 micron long) 10 fold from 5- 50 also increases the pain by 3 folds. (Ma Y et al., 2014) (Alvarez et al., 2001)
Fabrication of Microneedles
Microneedles can be fabricated employing micro-electromechanical systems (MEMS). The basic process can be divided in to three parts: deposition, patterning and etching. Deposition specially referred to the formation of thin films with a thickness anywhere between a few nanometers to about 100 micrometers. Patterning is the transfer of a pattern into the film. Lithography is used to transfer a pattern into a photosensitive material by selective exposure to a radiation source such as light. This process can involve photolithography, ion beam lithography, electron beam lithography. Diamond patterning is also a good option for lithography. Etching is a process of using strong acid or mordant to cut into the unprotected parts of a material’s surface to makes a design in it and can be divided into two categories: wet etching or dry etching. The selection of any of the above mentioned methods largely depends on the material of construction and the type of microneedles. Microneedles fabricated in different forms like as hollow, solid, and dissolving given below.
Hollow microneedles
Hollow microneedles contain a hollow bore in the centre of the needle. Hollow microneedles can be fabricated from a commercially available 30 gauge hypodermic needles. Pressure, and thereby flow rate, can be changed in hollow microneedles for a rapid bolus injection, a slow infusion or a varied delivery rate. Hollow microneedles can also be used to administer a larger dose of drug solution. When inserted into the skin, the hollow bore present by passes the stratum corneum layer of the skin and produces a direct channel into the other lower layers of the epidermis. The 4 × 4 pattern of holes was drilled in a polyetheretherketone mold (diameter 9 mm). Then, the needles were placed through the holes at a predetermined length of 300, 550, 700 and 900 μm. Subsequently, the needles were cut and glued at the back of the mold. A manual applicator was also designed for the microneedles array. (Donnelly et al., 2013)These microneedles are mainly employed to inject the drug solutions directly into the skin. These are very expensive to prepare and require expensive micro fabrication techniques. These micro needles contains hollow bore which offers possibility of transporting drugs through the interior of well defined needles by diffusion or for more rapid rates of delivery by pressure driven flow. Silicon microneedles are justified or described by their mechanical properties and their biocompatibility potential. However inconveniences such as high production costs or fragility have spurred researchers to look for other options. (Chen et al., 2007) Hollow silicon microneedles were fabricated by using isotropic etching followed by anisotropic etching to obtain a tapered tip. Silicon microneedles of 300 μm in height, with 130 μm outer diameter and 110 μm inner diameter at the tip followed by 80 μm inner diameter and 160 μm outer diameter at the base were fabricated using this technique. In order to improve the biocompatibility of microneedles, the fabricated microneedles were coated with titanium (500 nm) by sputtering technique followed by gold coating using electroplating. Hollow microneedles can fabricate using other system like micro-electro-mechanical systems technologies such as laser micromachining, deep reactive ion etching, integrated lithographic molding technique, and wet chemical etching and X-ray photolithography. AdminPen® microneedles have also been fabricated. These are hollow stainless steel microneedles of varying lengths from 600 to 1500 µm, which can be connected to a syringe and used to deliver liquid formulations. AdminStamp® devices contain AdminPatch® microneedle arrays attached to an applicator with six stainless steel screws. They can also be used to porate the skin without any liquid. When used in this way, they are like solid microneedles because they first create the holes before a drug solution is applied. (Sheer et al., 2011) Hollow microneedles can deposit a compound directly into the viable epidermis or the dermis avoiding the stratum corneum. This is especially useful for the delivery of high molecular weight compounds such as proteins, oligonucleotides and vaccines. Transdermal delivery of insulin continues to represent a significant scientific challenge. Cheung et al. used 1100 and 1400 µm long stainless steel microneedles to deliver insulin across porcine skin.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Solid Microneedles
Solid microneedles carried out passive diffusion for drug deliver by creating microchannels to increase skin permeability followed by the application of a drug-loaded patch on the channels. From a safety perspective, it is desirable for the microchannels to close soon after needle removal to prevent permeation of undesired toxic substances or infection by pathogenic microorganisms. Henry et al. used a deep reactive ion etching process to fabricate silicon microneedles and a chromium masking material was first deposited onto silicon wafers and patterned into dots which had a diameter approximately equal to the base of the desired microneedles. The wafers were then loaded into a reactive ion etcher and subjected to plasma etching. The regions protected by the metal mask remained to form the microneedles. Vinayakumar et al. fabricated an array of rectangular cup shaped silicon microneedles. These microneedles have the potential for reduced drug leakage resulting in improvement of drug delivery efficiency and the possibility of introducing multiple drugs. The fabricated solid microneedles with rectangular cup shaped tip are 200 μm in height. The cup shaped tips have dimensions of 60 × 60 μm (length × breadth) with a depth of 60 μm. The cups are filled with drug using a novel drop coating system. Solid microneedles fabricated from silicon, metal and polymer. (Nolan et al., 2003) (Bendas et al., 2007) Solid microneedles can be fabricated from polymers. Olatunji et al. prepared microneedles from biopolymer films extracted from fish scales of tilapia (Oreochromiss sp.) using micromolding technique.The microneedles were successfully inserted into porcine skin and were shown to dissolve gradually at 0, 60, 120 and 180 s after insertion. The microneedles contained methylene blue as model drug and successfully pierced porcine skin. (Bendas et al., 2007)
Dissolving Microneedles
Dissolving microneedles have a number of advantages. These include the one-step application process which is convenient for patients. Dissolving microneedles are fabricated on the basis of the “poke and release” principle. They are made from polysaccharides or other polymers. These microneedles release encapsulated drug into the skin following application and dissolution. Micromoulding is the preferred fabrication method for making dissolving microneedles. (Bendas et al., 2007)Certain drugs and vaccines are thermolabile so moulds are often filled with solutions of drugs and excipients and then dried under mild conditions. The fabrication process involves pouring the polymer solution into female molds, filling the microcavities of the mould under vacuum or pressure, drying under ambient conditions, centrifugation or pressure. (Kumar et al., 2011) Master structures for microneedles supporting arrays, and pressing tools were created by Chen et al. using proprietary electro-discharge-machining technology. Each master structure consisted of 64 (8 × 8) microstructures. Polydimethylsiloxane (PDMS) molds were created as exact inverse-replicates of the master structures. To prevent adhesion to PDMS molds, all master structures were sputter-coated with platinum. PDMS molds were fabricated by pouring PDMS solution over the master structure and allowing the polymer to cure overnight at room temperature. The cured PDMS molds were then peeled from the master structures and used to make the chitosan microneedles, polylactic acid supporting arrays, and polycaprolactone pressing tools.
Coated Microneedles
Coated microneedles refer to microneedles which have coating with the drug-containing dispersion. A plethora of techniques has been used in the literature to prepare coated microneedles. (Saroha et al., 2011) An approach using electrohydrodynamic atomization principles in the preparation of smart microneedles coatings was reported in the literature. Stainless steel (600–900 µm in height) microneedles were coupled to a ground electrode (in the electrohydrodynamic atomization coating set-up) with the deposition distance and collecting methodology varied for an ethanol: methanol (50:0) vehicle system. The authors used this technique to prepare nano- and micrometer-scaled pharmaceutical coatings. Fluorescein dye (serving as potential drug, sensory materials or disease state markers) and polyvinylpyrrolidone ,(polymer matrix system) formed the remaining components of the coating formulation. Based on these excipients and by varying the coating process, particles (100 nm to 3 µm) and fibres (400 nm to 1 µm) were deposited directly on microneedles in controlled and selectable fashion. (Tabassum et al., 2011)
Hydrogel-Forming Microneedles
The first two microneedle-based products, just recently marketed, Soluvia® and Micronjet®, are based on metal and silicon, respectively .However, the current trend in microneedle-based research has recognized biocompatibility problems associated with the use of silicon and the potential for inappropriate re-use of silicon or metal microneedles, which remain fully intact after removal from a patient’s skin. (Tabassum et al., 2011) This has led to a multiplicity of technologies aimed at overcoming this shortcoming. In this regard, recent effort has focused on microneedles formulated from aqueous polymer gels. One of these approaches involves the use of hydrogel-forming microneedles .One of the differences and advantages in comparison with regular dissolving polymer microneedles is that by using this drug delivery system, delivered doses of drugs and biomolecules are no longer limited to what can be loaded into the needles themselves. (Srinivas et al., 2010) Donnelly et al. prepared hydrogel-forming microneedles from “super-swelling” polymeric compositions. These are microneedle arrays, prepared under ambient conditions, which contain no drug themselves .Instead, they rapidly imbibe skin interstitial fluid upon insertion to form continuous conduits between the dermal microcirculation and an attached patch-type drug reservoir .Such microneedles act initially as a tool to penetrate the stratum corneum barrier. Upon swelling, they become a rate controlling membrane. Fluid uptake range in one hour was 0.9–2.7 μL which is of the same order of magnitude as the rates of interstitial fluid uptake for hollow microneedles and microdialysis. Other advantages of hydrogel-forming microneedles are that they can be fabricated in a wide range of patch sizes and geometries, can be easily sterilized, resist hole closure while in place and are removed completely intact from the skin. (Srinivas et al., 2010).
Evaluation Parameters (Vandervoort et al., 2008)
In vitro evaluation microneedles are accomplished by using various mediums like agarose gel and methanol to insert the microneedles. In vitro tests are used to determine the characteristics of new test device or compound. The main key objectives of the in vitro testing of microneedles involves optimization of the microneedles, finding out the penetration force and bending force, evaluation of strength of microneedles, determination of the dissolution rate of coating material and the estimation of the efficiency of drug delivery. Various methods employed for conducting in vitro studies are as follows:
Method 1
In vitro methods tested the delivery efficacy of the microneedles. In this test, the microneedles are integrated with Paradimethylsiloxane biochip and black ink is injected by the microneedles into the petridish, contains methanol widely used. The right triangular microneedles with 8.5 and 15 tip taper angles and isosceles triangular microneedles with 9.5 and 30 tip taper angles have been used for this purpose.
Method 2
In this method, the diluted form of Rhodamine B dye is injected through the microneedles into the 1% agarose gel to evaluate the penetration and flow of the solution after penetrating into the 1% agarose gel.
Method 3
Inserting microneedles into the porcine cadaver skin and pig cadaver skin for 10s to 20 s and 5 minutes respectively are evaluated by this method. This method is used to test the delivery efficacy, dissolution rate of the coated material, which is coated on the microneedle tip, coated with vitamin B, calcein or sulforhodamine. (Yadav et al., 2011)
In Vivo Testing of microneedles
To conduct the in vivo preclinical study, generally mice, rabbits, guinea pigs, mouse and monkey etc are used. The main motive of the in vivo testing is the determination of safety as well toxicity of the tested compound. The key objectives behind in vivo testing of the microneedles includes to perform skin toxicity test, determination of penetration force in different skin, mechanical stability, bending breakage force, to perform various non-clinical safety study and pharmacological study, determination of various parameters like immunogenicity, genotoxicity, skin sensitization and allerginisation, study, developmental toxicity, acute and chronic dermal toxicity, carcinogenicity. (Yadav et al., 2011) (Paik et al., 2003)
Method 1
This in vivo method involves testing of microneedles by pricking the microneedles into vein of the tail of hairless mice. It is used for the determination of the penetration force of the microneedles into the skin.
Method 2
This method of in vivo testing of the microneedles, Rhodamine B is injected into tail of laboratory mouse-tail and anaesthetized for the determination of penetration force and bending breakage force.
Method 3
This method has been performed for the evaluation of vaccine delivery via microneedles. Ovalbumin is used in this method, as a model protein antigen and administered into hairless guinea pig by using solid metal microneedles at the rate of 20 μg ovalbumin in 5s up to 80 μg.
Method 4
In this method rabbits have been used to evaluate the vaccine delivery. The anthrax vaccine containing recombinant protective antigen (rPA) of Bacillus anthracis has been administered in the rabbits via solid and hollow microneedles.
Clinical Trials and marketed product of microneedles (Jepps et al., 2013)
Microneedles were developed as a technology for administration of peptides, proteins, immunobiological, cosmetics and drugs as well as for biofluidic analysis. Intanza/IDflu is the first intradermal influenza vaccine marketed in Europe, Canada and Australia. Recently it was approved by the USFDA for marketing in the USA. ZP-PTH is a product developed by Zosano Pharma for the treatment of severeosteoporosis, which demonstrated excellent efficacy and safety in Phase II clinical trials and now is ready for Phase III study. Few examples of marketed products based on microneedle technology, together with their possible use given table. (Pearton et al., 2012) It would appear that many of the currently marketed applicators are utilized for improving the efficacy of cosmetic products. In 2009, a six-month, randomized, multicenter, blinded, multi-dose, Phase 2 clinical study using MN to deliver recombinant human parathyroid hormone 1-34, teriparatide was carried out. The product has completed Phase 2 clinical trials and is scheduled for Phase III trials. If approved, it will be used for the management of osteoporosis. The product is based on titanium microneedle arrays produced by photochemical etching. The MN come with a reusable applicator. NanoPass Technologies also has an intellectual property-backed product called MicronJet™. It is a single use, microneedle-based device for intradermal delivery of protein, drugs and vaccines.
Table 1. Marketed product of microneedles (Pearton et al., 2012)
|
Brand Name |
Manufacturer |
Application |
|
Micro-Trans
|
Valeritas Inc., USA |
It can easily deliver the drug into dermis without limitations of drug size, structure or the patient’s skin characteristics. |
|
Onvax Becton
|
Dickinson, USA |
It is a skin micro abrader having plastic microneedles for disruption of stratum corneum for the delivery of vaccines. |
|
AdminPen
|
AdminMed, USA |
Liquid pharmaceutical formulation or cosmetics can be conveniently injected into the skin. |
|
NanoCare,
|
NanoPass Inc. |
Israel It is a small hand-held device for rejuvenation of skin and to boosts the cosmetic effect of topical applications. |
Images of type of microneedles based on drug delivery (Dhamecha et al., 2009)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION
Microneedles either in the form of patch or an array have been observed as a potential carrier for the effective transdermal delivery for the delivery of numerous macromolecular drugs.
Various research reports studied confirmed that microneedles are ought to be the prominent carriers for enhancing the permeation deep into the systemic circulation and providing a painless, effective and safe route for the drug delivery. In future microneedles plays important role in innovation and design of controlled drug delivery for various drugs. These painless systems are slowly gaining importance and would qualify to be one of the important devices for controlled drug release in future. Thus, it was concluded that, these systems represented it to be an efficient and superior carriers as compared to other needle based formulation for the transdermal delivery.
REFERENCES
1. Alvarez-Figueroa M, Delgado-Charro M. Passive and iontophoretic transdermal penetration of methotrexate. Int J Pharm 2001; 212: 101-107.
2.Andrews S.N., Jeong E., Prausnitz M.R. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 2013;30:1099–1109. doi: 10.1007/s11095-012-0946-7. [PMC free article] [PubMed] [Cross Ref]
3.Arunachalam A, Karthikeyan M, Kumar M, Prathap M, Sethuraman S, Kumar SA, Manidipa S. Transdermal drug delivery system: a review. Curr Pharm Res 2010; 1(1): 70-81.
4.Bendas ER, Tadros MI. Enhanced transdermal delivery of salbutamol sulphate via ethosomes. AAPS PharmSciTech 2007; 8: 1-15.
5.Chen B, Wei J, Tay FEH, Wong YT, Iliescu C. Silicon micro needles array biodegradable tips for transdermal drug delivery. DTIP Mems Moems 2007; 1: 25-27
6.Chen M.-C., Huang S.-F., Lai K.-Y., Ling M.-H. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials. 2013;34:3077–3086. doi: 10.1016/j.biomaterials.2012.12.041. [PubMed] [Cross Ref]
7.Cheung K., Han T., Das D.B. Effect of Force of Microneedle Insertion on the Permeability of Insulin in Skin. J. Diabetes Sci. Technol. 2014;8:444–452. doi: 10.1177/1932296813519720. [PMC free article] [PubMed] [Cross Ref]
8.Dhamecha DL, Rathi AA, Saifee M, Lahoti SR, Dehghan MHG. Drug vehicle based approaches of penetration enhancement. Int J Pharm Pharma Sci 2009; 1(1): 24-46.
9.Donnelly R.F., Raj Singh T.R., Alkilani A.Z., McCrudden M.T.C., O’Neill S., O’Mahony C., Armstrong K., McLoone N., Kole P., Woolfson A.D. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: Potential for enhanced patient safety. Int. J. Pharm. 2013;451:76–91. doi: 10.1016/j.ijpharm.2013.04.045. [PMC free article] [PubMed] [Cross Ref]
10.Ehdaie B. Enhanced delivery of transdermal drugs through human skin with novel carriers. J Pharm Biomed Sci 2011;1(8): 161-166.
11.Gupta J., Gill H.S., Andrews S.N., Prausnitz M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control. Release. 2011;154:148–155. doi: 10.1016/j.jconrel.2011.05.021. [PMC free article] [PubMed] [Cross Ref]
12.Henry S., McAllister D.V., Allen M.G., Prausnitz M.R. Microfabricated microneedles: A novel approach to transdemal drug delivery. J. Pharm. Sci. 1998;87:922–925. doi: 10.1021/js980042+. [PubMed] [Cross Ref]
13.Hong X., Wei L., Wu F., Wu Z., Chen L., Liu Z., Yuan W. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des. Dev. Ther. 2013:945–952. [PMC free article] [PubMed]
14.Jepps O.G., Dancik Y., Anissimov Y.G., Roberts M.S. Modeling the human skin barrier—Towards a better understanding of dermal absorption. Adv. Drug Deliv. Rev. 2013;65:152–168. doi: 10.1016/j.addr.2012.04.003. [PubMed] [Cross Ref]
15.Khan H., Mehta P., Msallam H., Armitage D., Ahmad Z. Smart microneedle coatings for controlled delivery and biomedical analysis. J. Drug Target. 2014;22:790–795. doi: 10.3109/1061186X.2014.921926. [PubMed] [Cross Ref]
16.Kim Y.C., Park J.H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [PMC free article] [PubMed] [Cross Ref]
17.Kumar AV, Kulkarni PR, Raut RA. Microneedles: promising technique for transdermal drug delivery. Int J Pharm Bio Sci 2011; 2(1): 684-708.
18.Lhernould M.S., Deleers M., Delchambre A. Hollow polymer microneedles array resistance and insertion tests. Int. J. Pharm. 2015; 480:152–157. doi: 10.1016/j.ijpharm.2015.01.019.
19.Lhernould M.S., Deleers M., Delchambre A. Hollow polymer microneedles array resistance and insertion tests. Int. J. Pharm. 2015;480:152–157. doi: 10.1016/j.ijpharm.2015.01.019. [PubMed] [Cross Ref]
20.Ma Y., Gill H.S. Coating solid dispersions on microneedles via a molten dip-coating method: development and in vitro evaluation for transdermal delivery of a water-insoluble drug. J. Pharm. Sci. 2014;103:3621–3630. doi: 10.1002/jps.24159. [PMC free article] [PubMed] [Cross Ref]
21.Microsys 2003; 2: 1446-1449.
22.Nida Akhtar, Microneedles: An Innovative Approach to Transdermal Delivery. International journal of pharmacy and pharmaceutical sciences. Vol.6 issue 4,2014: 18-25.
23.Nolan LMA, Corish J, Corrigan OI, Fitzpatrick D. Iontophoretic and chemical enhancement of drug delivery: Part-I: Across Artificial Membranes. Int J Pharm 2003; 257: 41-55.
24.Olatunji O., Das D.B., Garland M.J., Belaid L., Donnelly R.F. Influence of array interspacing on the force required for successful microneedle skin penetration: Theoretical and practical approaches. J. Pharm. Sci. 2013;102:1209–1221. doi: 10.1002/jps.23439. [PubMed] [Cross Ref]
25.Paik SJ, Lim JM, Jung I, Park Y, Byun S, Chung S. A novel microneedle array integrated with a PDMS biochip for micro fluid system. Transducers Solid-State Sensors Actuators
26.Patel D, Chaudhary SA, Parmar B, Bhura N. Transdermal drug delivery system: a review. Pharm Innov 2012; 1(4): 66-75.
27.Pearton M., Saller V., Coulman S.A., Gateley C., Anstey A.V., Zarnitsyn V., Birchall J.C. Microneedle delivery of plasmid DNA to living human skin: Formulation coating, skin insertion and gene expression. J. Control. Release. 2012;160:561–569. doi: 10.1016/j.jconrel.2012.04.005. [PMC free article] [PubMed] [Cross Ref]
28.Prausnitz MR, Langer R. Transdermal Drug delivery. Nature Biotechnol 2008; 26: 1261-1268.
29.Ranade VV. Drug delivery systems: transdermal drug delivery. J Clin Pharmacol 1991; 31(5): 401-418.
30.Rani S, Saroha K, Syan N, Mathur P. Transdermal patches a successful tool in transdermal drug delivery system: an overview. Der Pharmacia Sinica 2011; 2(5): 17-29.
31.Saroha K, Nanda S, Rani S. Chemical penetration enhances: a novel approach in transdermal drug delivery system. Int J Curr Pharm Res 2011; 3(4): 5-9.
32.Sharma N, Bharat PS, Mahajan U. Blooming pharma indystry with transdermal drug delivery system. Indo Global J Pharm Sci 2012; 2(3): 262-278.
33.Sheer A, Chauhan M. Ethosomes as vesicular carrier for enhanced transdermal delivery of ketoconazole-formulation and evaluation. IJPI’s J Pharm Cosmetol 2011; 1(3): 1-14.
34.Srinivas P, Shanthi CL, Sadanandam MS. Miconeedles patches in drug delivery: a review. Int J Pharm Tech 2010; 2(3): 329-344.
35.Sullivan S.P., Koutsonanos D.G., del Pilar Martin M., Lee J.W., Zarnitsyn V., Choi S.-O., Murthy N., Compans R.W., Skountzou I., Prausnitz M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010;16:915–920. doi: 10.1038/nm.2182. [PMC free article] [PubMed] [Cross Ref]
36.Tabassum N, Sofi A, Khuroo T. Microneedle technology: a new drug delivery system. Int J Res Pharm Biomed Sci 2011; 2(1): 59-62.
37.Vandervoort J, Ludig A. Microneedles for transdermal drug delivery: a minireview. Front Biosci 2008; 1(13): 1711-1715.
38.Verbaan F.J., Bal S.M., van den Berg D.J., Dijksman J.A., van Hecke M., Verpoorten H., van den Berg A., Luttge R., Bouwstra J.A. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J. Control. Release. 2008;128:80–88. doi: 10.1016/j.jconrel.2008.02.009. [PubMed] [Cross Ref]
39.Vinayakumar K.B., Hegde G.M., Nayak M.M., Dinesh N.S., Rajanna K. Fabrication and characterization of gold coated hollow silicon microneedle array for drug delivery. Microelectron. Eng. 2014;128:12–18. doi: 10.1016/j.mee.2014.05.039. [Cross Ref]
40.Wang Q., Yao G., Dong P., Gong Z., Li G., Zhang K., Wu C. Investigation on fabrication process of dissolving microneedle arrays to improve effective needle drug distribution. Eur. J. Pharm. Sci. 2015;66:148–156. doi: 10.1016/j.ejps.2014.09.011. [PubMed] [Cross Ref]
41.Yadav JD. Microneedles: promising technique for transdermaldrug delivery. Int J Pharm BioSci 2011; 2(1): 684-708.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









