{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
S. Ashutosh Kumar*, Manidipa Debnath, Venugopal Padala,
Department of Pharmaceutical Analysis and Quality Assurance,
A.K.R.G College of Pharmacy, Nallajerla, West Godavari, A.P
*ashu.mpharm2007@gmail.com
ABSTRACT
Objective: The present work was undertaken with the aim to develop and validate a rapid and consistent RP-HPLC method in which the peaks will be appear with short period of time as per ICH Guidelines.
Method: The HPLC separation was achieved on an Inertsil-C18 ODS column (250 X 4.6 mm; 5 µ) column in an Isocratic Mode. The mobile phase composed of Methanol [HPLC Grade] (55 %) and Buffer (45 %) [pH 4.0 adjusted with triethylamine]. The flow rate was monitored at 1.0 mL/min. The wavelength was selected for the detection was 276 nm.
Results:The retention times found for rabeprazole and mosapride was 2.946 and 4.186 min respectively. The % recovery was 99.98- 100.03 for rabeprazole and 99.97 - 100.02 for mosapride. The linearity was established in the range of 20-80 µg/mL for both rabeprazole and mosapride. The LOD for rabeprazole and mosapride were 0.01 and 0.035 µg/mL respectively. The LOQ for rabeprazole and mosapride were 0.032and 0.11 µg/mL respectively.
Conclusion:The proposed method was adequate sensitive, reproducible, and specific for the determination of rabeprazole and mosapride in bulk as well as in tablet dosage forms.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2422
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 7 Received On: 27/02/2016; Accepted On: 08/03/2016; Published On: 01/07/2016 How to cite this article: Kumar SA, Debnath M, Padala V; An Iso cratic Method Development and Validation for simultaneous estimation of Rabeprazole and Mosapride in Tablet Dosage Forms by using RP-HPLC; PharmaTutor; 2016; 4(7); 41-51 |
INTRODUCTION
Rabeprazole sodium is chemically (RS)-2-[(4-(3-methoxy propaxy)-3-methylpyridin-2-yl] methyl sulphonyl)-1H-benzo (d) imidazole (fig. 1). Rabeprazole sodium[1] is an antiulcer drug in the class of proton pump inhibitors. As anti ulcer drug, it is used in short-term treatment in healing and symptomatic relief of duodenal ulcers and erosive or ulcerative gastro esophageal reflux disease (GORD); maintaining healing and reducing relapse rates of heartburn symptoms in patients with GORD; treatment of daytime and nighttime heartburn and other symptoms associated with GORD; long-term treatment of pathological hypersecretory conditions, including Zollinger-Ellison syndrome and in combination with Amoxicillin and Clarithromycin to eradicate H. pylori. Mosapride citrate is chemically (RS)-4-amino-5-chloro-2-ethoxy-N-[(4-(4-fluorobenzyl) morpholin-2-yl) methyl] bezamide citrate (fig. 2). Mosapride is a gastro pro-kinetic agent that acts as a selective 5HT4 agonist. The major active metabolite of Mosapride is known as M1, additionally acts as a 5HT3 antagonist. In addition to its prokinetic properties, Mosapride also exerts anti-inflammatory effects on GIT which may contribute to some of its therapeutic effects. Mosapride also promotes neurogenesis in the gastrointestinal tract which may prove useful in certain bowel disorders. The neurogenesis is due to Mosapride’s effect on 5-HT4 receptor where it acts as an agonist. The drug analysis plays an important role in the development of drugs, manufacturing and therapeutic use. Pharmaceutical industries rely upon quantitative chemical analysis to ensure that the raw material used and the final product obtained meets the required specification.

Fig. no. 1 It shows the chemical structure of Rabeprazole

Fig. no. 2 It shows the chemical structure of Mosapride
The literature review indicates there are several analytical methods have been reported for estimation of these drugs as individual or in combination with other drugs, and also several analytical methods for the determination of simultaneous estimation of Rabeprazole sodium and Mosapride citrate by RP-HPLC[2-9], Spectrofluorimetry, thin layer chromatography and column high-performance liquid chromatography[10], Spectrophotometric and chromatographic[11-12], HPTLC[13] and TLC[14] in dosage formulation and/or in presence of its degraded products. Some of the reported RP-HPLC methods were not economical in terms of mobile phase composition, column dimensions and run times. Hence there is need for the development of newer method for estimation of Rabeprazole sodium and Mosapride citrate present in tablet to overcome above discussed hurdles. In addition, RP-HPLC method for the simultaneous estimation of Rabeprazole sodium and Mosapride citrate in pharmaceutical dosage form are very scanty. Hence the main objective of this study is to develop a RPHPLC method for estimation of Rabeprazole sodium and Mosapride citrate and validate the developed method according to ICH guidelines[15-19] by using various parameters.
MATERIALS AND METHODS
Chemicals and Reagents Used: The following chemicals were used for the processwater [HPLC Grade], rabeprazole and mosapride [working standards] gift samples collected from Pharma Train Lab., Hyderabad, Telagana., methanol [HPLC Grade], ammonium acetate and triethylamine. All the chemicals were procured from Standard Solutions, Hyderabad, Andhra Pradesh.
0.45 µ membrane filters (Advanced Micro Devices Pvt. Ltd., Chandigarh, India) were used for filtration of various solvents and solutions intended for injection into the column.
Apparatus and Chromatographic Conditions: The equipment used was High Performance Liquid Chromatography Equipped with Auto Sampler and DAD or UV Detector. The column Inertsil-C18 ODS column (250 X 4.6 mm; 5 µ) was selected. The flow rate was monitored at 1.0 mL/min. The detection was carried out at 276 nm. The injection volume selected 20 µL, the temperature of the column oven was maintained at 25 °C, the detector used was Photo diode array and the run time was 10.0 min.
The ultra violet spectra of the drugs used for the investigation were taken on a Lab India UV 3000 spectrophotometer for finding out their λmax values.
Solubility of the compounds was enhanced by sonication on an ultra sonicator (Power Sonic 510, (Hwashin Technology).
All the weighings in the experiments were done with an Afcoset electronic balance. The Hermle microlitre centrifuge Z100 (model no 292 P01) was used for the centrifugation process and Remi equipments (model no- CM101DX) Cyclomixer was used.
Glassware: All the volumetric glassware used in the study was of Grade A quality Borosil.
Preparation of Phosphate buffer[20]:The buffer solution was prepared by weighing accurately 3.85 gm of ammonium acetate and transferred to a clean and dry 1000 mL volumetric flask. Initially, about 900 mL of water [HPLC grade] was added. The final volume was made upto the mark with water. Then the pH was adjusted to 4.0 with triethylamine.
Preparation of mobile phase: The mobile phase was prepared by mixing a mixture of above buffer 450 mL (45 %) and 550 mL of methanol HPLC (55 %) and degas in ultrasonic water bath for 5 minutes. Then, the resultant solution was filtered through a 0.45 µ filter under vacuum.
Preparation of standard solution of Rabeprazole and Mosapride: About 10 mg rabeprazole was weighed accurately and transferred into a 10 mL clean and dry volumetric flask. Initially, the drug was mixed with 7 mL of diluent. The solution was sonicated for 15 min for complete dissolution of the drug. The final volume was made up to the mark with the same solvent. Similarly, about 10 mg mosapride was weighed accurately and transferred into a 10 mL clean and dry volumetric flask. Initially, the drug was mixed with 7 mL of diluent. The solution was sonicated for 15 min for complete dissolution of the drug. The final volume was made up to the mark with the same solvent to get a concentration of 1000 µg/mL.
From the above prepared stock solutions 0.4 mL of rabeprazole and mosapride were pipetted out into a 10 mL clean and dry volumetric flask and it was diluted up to the mark with diluent. This mixed stock solution contains 40.0 µg/mL of rabeprazole and 40.0 µg/mL of mosapride.
Preparation of sample solution of Rabeprazole and Mosapride: Twenty tablets were weighed accurately and a quantity of tablet powder equivalent to 20 mg of rabeprazole and 20 mg of mosapride were weighed and dissolved in the 70 mL mobile phase with the aid of ultra sonication for 20 min. The content was diluted with 100 mL mobile phase to furnish the preparation of stock solution. The stock solution was filtered through a 0.45 µm Nylon syringe filter and 10.0 mL of the filtrate was diluted into a 50.0 mL volumetric flask to get the desired concentration of 40.0 µg/mL of rabeprazole and 40.0 µg/mL of mosapride.
System Suitability: The tailing factor for the peaks due to rabeprazole and mosapride in Standard solution should not be more than 2.0. The Theoretical plates for the rabeprazole and mosapride peaks in Standard solution should not be less than 2000.The system suitability of the method was checked by injecting five different preparations of the rabeprazole and mosapride. The parameters of system suitability were checked.
[adsense:468x15:2204050025]
VALIDATION DEVELOPMENT[15-19]
1. System Suitability:A Standard solution was prepared by using rabeprazole and mosapride working standards as per test method and was injected Five times into the HPLC system. The system suitability parameters were evaluated from standard chromatograms by calculating the % RSD from five replicate injections for rabeprazole and mosapride, retention times and peak areas. The data are represented in table no. 1 and 2.
Acceptance Criteria: The % RSD for the retention times of principal peak from 5 replicate injections of each Standard solution should be not more than 2.0 %. The number of theoretical plates (N) for the Sumatriptan succinate and Naproxen sodium peaksis NLT 3000. The Tailing factor (T) for the Sumatriptan succinate and Naproxen sodium peaks is NMT 2.0.
Table no. 1: It shows the system suitability data for Rabeprazole
|
Injection |
RT |
Peak Area |
USP Plate count |
USP Tailing |
|
1 2 3 4 5 Mean SD % RSD |
2.951 2.950 2.948 2.949 2.949 2.947 0.003701 0.125589 |
2120053 2120059 2120201 2120054 2120451 2120164 172.6146 0.01 |
11898.457087 11844.975123 11857.288976 11809.408109 11669.365498 11815.9 ------- ------- |
1.214954 1.215568 1.207595 1.217034 1.214530 1.213936 ------- ------- |
Table no. 2: It shows the system suitability data for Mosapride
|
Injection |
RT |
Peak Area |
USP Plate count |
USP Tailing |
|
1 2 3 4 5 Mean SD % RSD |
4.195 4.193 4.189 4.190 4.188 4.191 0.002828 0.067488 |
1440041 1440064 1440420 1440309 1440984 1440364 382.3902 0.03 |
9559.400562 9468.102886 9470.850282 9425.185779 9253.320313 9435.372 ------- ------- |
1.141374 1.136440 1.146321 1.147756 1.145364 1.143451 ------- ------- |
2. Specificity: Solutions of standard and sample were prepared as per the test method are injected into chromatographic system. The chromatograms of standard and sample should be identical with near retention time. The specificity for method is represented in fig.no.3 and 4.
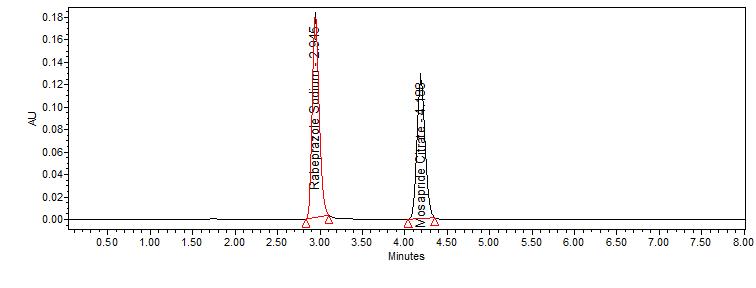
Fig. No. 3: It shows a typical chromatogram for standard drugs

Fig. No. 4: It shows a typical chromatogram for sample drugs
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3. Precision: It is a measure of degree of repeatability of an analytical method under normal operation and it is normally expressed as % of relative standard deviation (% RSD). The standard solution was injected for five times and measured the area for all five injections in HPLC. The % RSD for the area of five replicate injections was found to be within the specified limits. The data are represented in table no. 3 and 4.
Table no. 3: It shows precision results for Rabeprazole
|
Injection |
Peak Areas of Rabeprazole sodium |
% Assay |
|
1 2 3 4 5 6 Mean SD % RSD |
2120053 2120201 2120451 2120304 2120409 2120059 2120246 170.9976 0.01 |
99.98 99.99 100.00 99.99 100.00 99.98 99.99 0.008 0.01 |
Table no. 4: It shows precision results for Mosapride
|
Injection |
Peak Areas of Mosapride |
% Assay |
|
1 2 3 4 5 6 Mean SD % RSD |
1440041 1440420 1440984 1440452 1440657 1440064 1440436 358.9054 0.02 |
99.97 100.01 100.04 100.00 100.01 99.97 100 0.026 0.02 |
Acceptance Criteria:The %RSD for the area of all the five injections should not be more than 2%.
4. Intermediate Precision / Ruggedness: To evaluate the intermediate precision (also known as Ruggedness) of the method, precision was performed on different day by using different make column of same dimensions. The standard solution was injected for five times and measured the area for all five injections in HPLC. The % RSD for the area of five replicate injections was found to be within the specified limits. The data are represented in table no. 5 and 6.
Table no. 5: It shows ruggedness results for Rabeprazole
|
Injection |
Peak Areas of Rabeprazole sodium |
% Assay |
|
1 2 3 4 5 6 Mean SD % RSD |
2120451 2120304 2120409 2120059 2120054 2120199 2120246 170.8801 0.01 |
100.00 99.99 100.00 99.98 99.98 99.99 99.99 0.008 0.01 |
Table no. 6: It shows ruggedness results for Mosapride
|
Injection |
Peak Areas of Mosapride |
% Assay |
|
1 2 3 4 5 6 Mean SD % RSD |
1440984 1440452 1440657 1440064 1440309 1440379 1440474 315.7286 0.02 |
100.04 100.00 99.97 99.97 99.99 99.99 99.99 0.02 0.02 |
Acceptance Criteria:The %RSD for the area of all the five injections should not be more than 2%.
5. Accuracy: The accuracy of an analytical procedure expresses the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and value found. The standard solution with Accuracy -50 %, Accuracy -100 % and Accuracy -150 % were injected into chromatographic system and calculated the amount found and amount added for Rabeprazole and Mosaprideand further calculated the individual recovery and mean recovery values. The data are represented in table no. 7 and 8.
Table No. 7: It shows accuracy results for Rabeprazole
|
Concentration % of spiked level |
Amount added (mg) |
Amount found (mg) |
% Recovery |
Statistical Analysis of % Recovery |
|
|
50 % 50 % 50 %
|
20 20 20 |
20.00 19.99 20.01 |
100.00 99.99 100.03 |
MEAN |
100.00 |
|
|
|
||||
|
% RSD |
0.02 |
||||
|
100 % 100 % 100%
|
40 40 40 |
39.99 39.99 39.99 |
99.98 99.98 99.99 |
MEAN % RSD |
99.98 0.01 |
|
150 % 150 % 150 % |
60 60 60 |
59.99 59.99 59.99 |
99.99 99.99 99.98 |
MEAN %RSD |
99.98 0.01 |
Table No. 8: It shows accuracy results for Mosapride
|
Concentration % of spiked level |
Amount added (mg) |
Amount found (mg) |
% Recovery |
Statistical Analysis of % Recovery |
|
|
50 % 50 % 50 % |
20 20 20 |
19.99 19.99 20.01 |
99.98 99.98 100.06 |
MEAN %RSD |
100.00 0.04 |
|
100 % 100 % 100 % |
40 40 40 |
40.00 39.99 40.00 |
100.00 99.97 100.00 |
MEAN %RSD |
99.99 0.01 |
|
150% 150 % 150 % |
60 60 60 |
60.01 59.99 60.01 |
100.02 99.99 100.02 |
MEAN %RSD |
100.01 0.01 |
Acceptance Criteria:The %Recovery for each level should be between 98.0 to 102.0 %.
6. Linearity:It is the ability of the method to elicit test result that is directly proportional to analytic concentration within a given range. It is generally reported as variance of slope or regression line. It is determined by series of three to six injections of five of more standards. Different levels of solution were prepared and injected to the chromatographic system and the peak area was measured.Plotted a graph of peak area versus concentration (on X-axis concentration and on Y-axis Peak area) and calculate the correlation coefficient. The calibration curve was represented in fig. no. 3 and 4. The data are represented in table no. 5 and 6.
Table no. 9: It shows linearity results for Rabeprazole
|
Concentration (µg/mL) |
Average Area |
Statistical Analysis |
|
|
20 30 40 50 60 70 80 |
1060367 1590454 2120164 2650519 3180207 3710698 4240367 |
Slope y-Intercept Correlation Coefficient |
53002 301.7 1 |
Table no. 10: It shows linearity results for Mosapride
|
Concentration (µg/mL) |
Average Area |
Statistical Analysis |
|
|
20 30 40 50 60 70 80 |
720468 1080643 1440364 1800465 2160760 2520583 2879905 |
Slope y-Intercept Correlation Coefficient |
35995 707.7 1 |

Fig. no. 5: It shows calibration curve for Rabeprazole
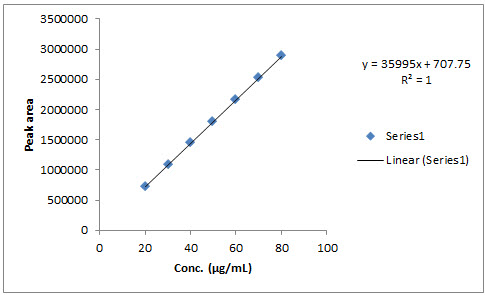
Fig. no. 6: It shows calibration curve for Mosapride
7. Limit of Detection: The detection limit of an individual analytical procedure is the lowest amount of analyte in a sample which can be detected but not necessarily quantities as an exact value.
Limit of Detection for Rabeprazole and Mosapride: The lowest concentration of the sample was prepared with respect to the base line noise and measured the signal to noise ratio. Limit of detection is the lowest concentration of the substance that can be detected, not necessarily quantified by the method. (Regression statistics)The minimum concentration at which the analyte can be detected is determined from the linearity curve by applying the following formula.
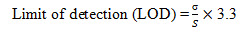
Where S – slope of the calibration curve
σ – Residual standard deviation
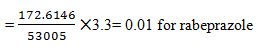
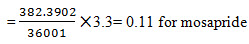
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
8. Limit of Quantification: It is defined as lowest concentration of analyte in a sample that can be determined with acceptable precision and accuracy and reliability by a given method under stated experimental conditions. LOQ is expressed as a concentration at a specified signal to noise ratio.
Limit of Quantification for Rabeprazole and Mosapride:The lowest concentration of the sample was prepared with respect to the base line noise and measured the signal to noise ratio. Limit of Quantificationis the lowest concentration of the substance that can be estimated quantitatively. It can be determined from linearity curve by applying the followingformula
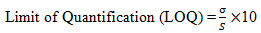
Where S – slope of the calibration curve
σ – Residual standard deviation
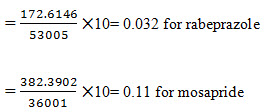
9. Robustness: As part of the Robustness, deliberate change in the flow rate, mobile phase composition, temperature variation was made to evaluate the impact on the method. The standard and samples of Rabeprazole and Mosapride were injected by changing the conditions of chromatography. There was no significant change in the parameters like resolution, tailing factor, asymmetric factor, and plate count.The data are represented in table no. 11 and 12 and fig. no. 7, 8 and 9.
Table No. 11: It shows the system suitability results for Rabeprazole(Change in Flow Rate)
|
Flow 0.8 mL/min. |
Std Area |
Tailing factor |
Flow 1.0 mL/min. |
Std Area |
Tailing factor |
Flow 1.2 mL/min. |
Std Area |
Tailing factor |
|
|
2588404 2588146 2588507 2588340 2588295 |
1.212666 1.231926 1.219733 1.218720 1.217223 |
|
2120053 2120059 2120201 2120054 2120451 |
1.214954 1.215568 1.207595 1.217034 1.214530 |
|
1730893 1730892 1730548 1730620 1730742 |
1.289723 1.284669 1.285484 1.284423 1.285398 |
|
Avg. SD % RSD |
2588338 133.8219 0.01 |
1.220054 0.007166 0.57 |
Avg. SD % RSD |
2120164 172.6146 0.01 |
1.213936 0.003 0.25 |
Avg. SD % RSD |
1730739 156.3458 0.01 |
1.285939 0.002 0.16 |
Table No. 12: It shows the system suitability results for Mosapride(Change in Flow Rate)
|
Flow 0.8 mL/min. |
Std Area |
Tailing factor |
Flow 1.0 mL/min. |
Std Area |
Tailing factor |
Flow 1.2 mL/min. |
Std Area |
Tailing factor |
|
|
1919212 1919607 1918031 1919556 1919620 |
1.144564 1.134991 1.130453 1.135498 1.134825 |
|
1440041 1440064 1440420 1440309 1440984 |
1.141374 1.136440 1.146321 1.147756 1.145364 |
|
1291932 1291600 1291369 1291294 1291498 |
1.286913 1.303066 1.313891 1.303122 1.303542 |
|
Avg. SD % RSD |
1919205 677.3763 0.035 |
1.136066 0.005166 0.45 |
Avg. SD % RSD |
1440364 382.3902 0.02 |
1.143451 0.00458 0.35 |
Avg. SD % RSD |
1291539 249.3868 0.01 |
1.302107 0.009 0.69 |

Fig. no. 7: It shows typical chromatogram for robustness with flow rate
(for 0.8 mL/min flow)

Fig. no. 8: It shows typical chromatogram for robustness with flow rate
(for 1.0 mL/min flow)
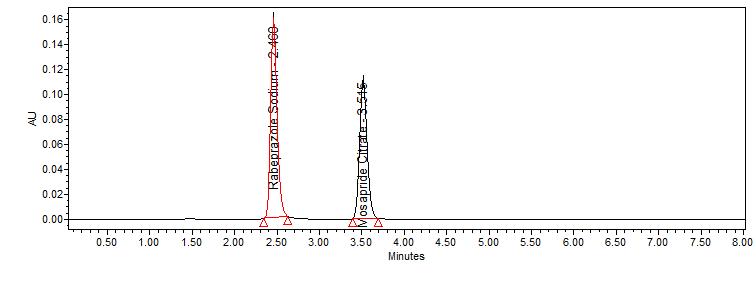
Fig. no. 9: It shows typical chromatogram for robustness with flow rate
(for 1.2 mL/min flow)
RESULTS AND DISCUSSION
To optimize the mobile phase, various proportions of ammonium acetate buffer (pH 4.0) with methanol [HPLC Grade] were tested. The use of ammonium acetate buffer (pH 4.0) and methanol [HPLC Grade] in the ratio of 45:55 (v/v) resulted in peak with good shapes and resolution. A flow rate of 1.0 mL /min was found to be optimum in the 0.4-1.5 mL/min range resulting in short retention time, baseline stability and minimum noise.
By applying the proposed method, the retention times of rabeprazole and mosapride were observed at 2.946 and 4.186 minat 276 nm respectively. A typical chromatogram is represented in fig. no. 10.

Fig. No. 10 It shows typical chromatogram for Rabeprazole and Mosapride
Quantitative linearity was obeyed in the concentration ranges of 20-80 µg/mL for both rabeprazole and mosapride. The relevant regression equations were y = 53002x + 301.7 for rabeprazole (r2= 1) and y = 35995x + 707.7 for mosapride (r2= 1) (where y is the peak area ratio and x is the concentration of rabeprazole and mosapride (µg/mL)). The intra-day and inter-day drugs variations by the proposed method showed an RSD less than 2 %, indicating that the method is precise. The corresponding mean recoveries of the drugs were 99.98- 100.03 % for rabeprazole and 99.97 - 100.02 % for mosapride. This reveals that the method is quite accurate. The tailing factor (1.21 and 1.14 for rabeprazole and mosapride), USP plate count (11815.9 and 9435.372 for rabeprazole and mosapride); obtained were within the acceptance limits. The limits of detection for rabeprazole and mosaprideobtained by the proposed method were 0.01 and 0.035 µg/mL respectively, and limits of quantification for atorvastatin and ezetimibe obtainedby the proposed method were 0.032and 0.11 µg /mL respectively, which indicate the sensitivity of the method. The method tolerated minor variations in optimized chromatographic conditions indicating good robustness, which indicate the efficient performance of the column.
No interfering peaks were found in the chromatograms indicating that the excipients used in tablet formulations did not interfere with the estimation of the drug by the proposed HPLC method.
CONCLUSION
The proposed HPLC method was found to be simple, precise, accurate and sensitive for the simultaneous determination of rabeprazole and mosapride. The method was validated as per ICH guidelines and all the parameters met within the acceptance criteria. Applicability of this method for simultaneous estimation of rabeprazole and mosapridefrom tablet dosage forms was confirmed. Hence, this method is specific and can be successfully used for the simultaneous estimation of rabeprazole and mosapridein bulk drug samples, pharmaceutical dosage forms. Hence, this method can be easily and conveniently adopted for routine quality control analysis of the above drugs.
ACKNOWLEDGEMENT: The authors greatly acknowledge M/s. Pharma Train, Hyderabad, Telagana, India for providing the gift sample of rabeprazole and mosapride.
REFERENCES
1. Carswel C. I. and Goa K L; Rabeprazole: an update of its use in acid related disorders; Drugs 2001; 61(15); 2327-2356.
2. Bhavesh Patel H., Madhabhai Patel M., Jignesh Patel R. and Bahnubhai Suhagia N; HPLC analysis for simultaneous determination of Rabeprazole and Domperidone in pharmaceutical formulation; J. Liq. Chrom. Rel. Tech. 2007; 30(3); 439-445.
3. Prasanna B. R. and Reddy M. S; Development and validation of RP-HPLC for the determination of Rabeprazole sodium in pharmaceutical formulations and human plasma; Asian J Res Chem; 2009; 2(1); 495-499.
4. Garcia CV, Paim C.S. and Steppe M; New liquid chromatographic method for determination of Rabeprazole sodium in coated tablets; J AOAC Int; 2004; 87(4); 842-846.
5. Rajesh S., Ganesh P. M., Subhash C. C; Development and validation of RP-HPLC method for the simultaneous determination of Rabeprazole sodium and Itopride hydrochloride in solid dosage form; E J Chem.; 2010; 7(3); 947-952.
6. Pillai S. and Singhvi I; Quantitative estimation of Itopride hydrochloride and Rabeprazole sodium from capsule formulation; Indian J Pharm Sci.; 2008; 70(5); 658-661.
7. Cassia Garcia V., Norma Nudelman S., Martin Steppe. and Elfrides Schapoval E.S; Structural elucidation of Rabeprazole sodium photo degradation products; J. Pharm Biomed Ana; 2008; 46(1); 88-93.
8. Patel B. H., Suhagia B. N. and Patel M. M; High-performance liquid chromatography and thin-layer chromatography for the simultaneous quantitation of Rabeprazole and Mosapride in pharmaceutical products; J Chrom Sci; 2008; 46(1); 4-10.
9. Shan Ren., Mi-Jin Park., Hongkee Sah. and Beom-Jin Lee; Effect of pharmaceutical excipients on aqueous stability of Rabeprazole sodium; Int J Pharm; 2008; 350(1-2); 197-204.
10. Osman A. O; Spectrofluorimetry, thin layer chromatography and column high-performance liquid chromatography determination of Rabeprazole sodium in the presence of its acidic and oxidized degradation products; J AOAC Int.; 2009; 92(5); 1373-1381.
11. El-Gindy A., El-Yazby F. and Maher M. M; Spectrophotometric and chromatographic determination of Rabeprazole in presence of its degradation products; J Pharm Biomed Anal. 2003; 31(2); 229-242.
12. Pattanayak P., Sharma R. and Chaturved S. C; Simultaneous spectrophotometric estimation of Rabeprazole sodium and Itopride HCl; Anal Lett; 2007; 40(12); 2288-2294.
13. Suganthi A., Sofiya J. and Ravi T. K; Simultaneous HPTLC determination of Rabeprazole and Itopride hydrochloride from their combined dosage form. Indian J Pharm Sci; 2008; 70(3); 366-8.
14. Shirkhedkar A. A. and Surana S. J; Application of stability-indicating RP-TLC densitometric determination of Rabeprazole sodium in bulk and pharmaceutical formulation; Eurasian J Anal Chem; 2009; 4(1); 165-170.
15. US Food and drug administration; Guidance for industry: Q2B validation of analytical procedures: methodology, Rockville; 1996.
16. US FDA; Guideline for industry: text on validation of analytical procedures: ICH Q2A; Rockville, MD; 1995.
17. International conference on harmonization (ICH), ICH quality guidelines: Good manufacturing practice guidance for active pharmaceutical ingredients Q7A (ICH), Geneva, Switzerland; 2001.
18. US Food and drug administration; Guidance document for industry; Analytical procedures and methods validation, FDA, Rockville, MD; 2000.
19. ICH, ICH Quality guidelines validation on analytical procedures: Methodology Q2B, ICH, Geneva, Switzerland; 1996.
20. Indian Pharmacopeia 2007; Volume I; Published by The Indian Pharmacopoeia Commission; 477-478.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









