ABOUT AUTHOR
Rajesh Vagh
PharmaTutor Edu Labs
Surat, Gujarat, India
So far, medical representatives are assets for pharma sales but need of time and competitions are asking for variant ways to promote their products. Patient portals, apps and online communities should be developed with more intensity and utilize technologies to improve customer experience.
Spending of pharmaceutical industries on digital advertising is below par that of other industries, and they still conducts most of their communications with physicians via traditional channels. There is huge potential for new communications through digitalization to make the pharmaceutical industries more efficient and more engaged with their customers. Due of the nature of the industry there are more rules and regulations, which initially caused a slower uptake in digital marketing compared with some other industries.
The pace of transformation has increased, competition has intensified and business models have been profoundly disrupted. This shift is happening at breakneck speed across industries, and pharma can no longer be an exception. Customers have already embraced technological changes, through their many digital touch points, and pharma must look toward digital to reimagine the customer experience. The urgency of acting is acute. It is time that pharma companies in India took a step back and re-envisioned digital as a core strategic enabler.
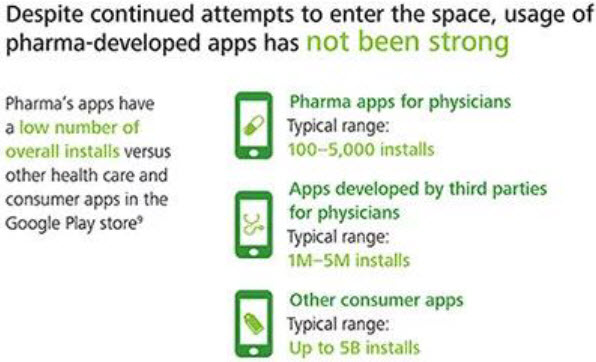
PATIENT COMMUNICATION
Traditionally, pharmaceutical industries develop all sales strategies circle around physicians, potential prescribers. But, what about end users? In Pharma, we call them patients. Patients are those who will consume their products or medicines. Mobile revolution created bigger hike in India and numbers of smart phone users is increasing day by day. Now, patients are searching disease conditions, prescribed medicines details, pricing etc. online and this will be the bull’s eye for pharmaceutical industries. So far, medical representatives are assets for pharma sales but need of time and competitions are asking for variant ways to promote their products. Patient portals, apps and online communities should be developed with more intensity and utilize technologies to improve customer experience.
So far, medical representatives are assets for pharma sales but need of time and competitions are asking for variant ways to promote their products.
BE SERVICE PROVIDER, NOT JUST PILLS
Pharmaceutical companies should not to be just pill sellers but they need to create relationship with patients for better patient compliance and it is possible through development of proper patient centered portals or cropped digital tools where they can even enroll physicians and pharmacists along with them. Online communication policy can be shown in a simple model adapted from Kotler's Marketing Process Model, where prior four stages act to understand patients and their needs, patients class, create patient value and build strong patient relationships and the last stage, they bring back the profit of creating superior value.
Novartis's ViaOpta app is a perfect example for it. This app allows a blind/low vision person to move independently by guiding them to walk and have information useful to facilitate their orientation while moving.

Internet can be source of disoriented, obscure and unauthentic information. And in case of diseases and medicines, this can be life-threatening for patients. Pharmaceutical companies can be trusted and authentic source of information for patients which ultimately patients will develop trust factor for company and it’s brand.
REAL TIME DIAGNOSTIC DATA AND ADHERENCE
Medication non-adherence leads to uncontrolled health conditions, excess hosipitalizations, emergency room visits and office visits, resulting in additional cost to the U.S. healthcare system of $290 billion annually. I really don’t found Indian data on that but I am sure it is much higher in India. Non-adherence is multifactorial and must be addressed by understanding the underlying cause (e.g. side effects, lack of understanding of the disease, and dosing schedule). Accurate and timely adherence data necessary to diagnose non-adherence and its root cause and then allow the patient and provider to respond quickly to poor adherence. This may seem like magic to some of you but it is possible now. And here is, Proteus Discover.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Proteus Discover is the world’s first digital medicine approved by FDA in 2012. It is designed for health organizations searching for efficient, patient centric approach to measure, manage and improve health of patients. It consists of an ingestible sensor the size of a grain of sand, a small wearable sensor patch, an application on a mobile device and a provider portal. The patient activates Proteus Discover by taking medication with an ingestible sensor. Once the ingestible sensor reaches the stomach, it transmits a signal to the patch worn on the torso. A digital record is sent to the patient’s mobile device and then to the Proteus cloud where with the patient’s permission, healthcare providers and caregivers can access it via their portal. The patch also measures and shares patient activity and rest. Recently, in May 2017, Otsuka Pharmaceutical and Proteus Digital Health® has resubmitted the New Drug Application (NDA) with FDA for the drug-device combination product of ABILIFY® (aripiprazole) embedded with a Proteus ingestible sensor in a single tablet.

In 2015, Verily (formerly Google Life Sciences) filed a patent for a needle free blood draw device at US Patent and Trademark Office. This device designed for people who are frequently needed to test their blood. Google is already in collaboration with Novartis, working on smart lenses that will analyze tear to sense blood glucose level and provide real time data. On one step ahead, Google is also developing cloud-connected sensor which can monitor blood sugar.
Don’t you think such new innovations can change the healthcare revenue system ? Write your feedback!
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









