About Author
KONDEPATI MALLESH BABU
Priyadarshini Institute of Pharmaceutical Education and Research
kondepatimallesh@gmail.com
ABSTRACT
Gene therapy is defined because the ability to improve genes thru the alteration of genes or site-specific adjustments for the reason of treatment. The disorder and its genetic interactions Gene therapy process: gene release every day gene are inserted into the genome to update unusual genes that reason positive diseases. Cystic fibrosis is a lifestyles-threatening autosomal-recessive disease because of mutations in an unmarried gene encoding cystic fibrosis transmembrane conductance regulator CFTR deficiency is a drug discovery goal and its improvements characteristic of CFTR results in ailment development those ensuing phenotypes have reignited interest and investment in different therapeutics focused on molecular, defects, consisting of gene remedy.
INTRODUCTION
A gene is a genetic unit made up of a segment of DNA or a chromosome located at a certain locus (gene locus) and includes information about crossing and mutations. RNA can also carry information from generation to generation and is usually the only domain of a DNA enzyme or protein, one for each gene The distinction between the two strategies is that during somatic gene remedy, the genetic fabric is brought into unique target cells. everyday gene are inserted into the genome to update unusual genes that reason positive diseases. some of the diverse challenges associated with this system, one of the primary ones is Gareth’s issue of freeing genes into stem cells. consequently, molecular carriers known as 'vectors' are used to deliver the gene. Angelman Syndrome (AS is an unprecedented neurodevelopmental disorder characterized by using excessive developmental put-off. intellectual incapacity, negative verbal exchange abilities, high occurrence of seizures, sleep disturbances, ataxia, Motor abnormalities, and microcephaly UBE3A encodes a hundred kDa ubiquity in-protein ligase. This process occurs within the germline wherein methyl companies are connected to DNA inside fragments. rich in cytosine guanine dinucleotide, the Formation of an imprinting area regulating expression within the q11–thirteen areas of chromosome 15 contains a cluster of imprinting genes, inclusive of UBE3A, which has maternal or paternal expression and is associated with numerous neurodevelopmental problems.
GENE
Genes are genetic components that are passed down from parents to children. A gene is a genetic unit made up of a segment of DNA or a chromosome located at a certain locus (gene locus) and includes information about crossing and mutations. When biochemistry was in full swing, these properties associated with RNA molecules were not only passive channels through which genetic messages circulated in the world but also active regulators of cellular processes. In some cases, RNA can also carry information from generation to generation and is usually the only domain of a DNA enzyme or protein, one for each gene1. A gene is:
(i) a unit of reproductive genetic material.
(ii) recombination unit is, i.e., can be crossed over;
(iii) A unit of genetic material susceptible to mutation.
(iv) genetic units associated with physical structure or function that result in phenotypic expression. Levin (2000) defined a gene as a DNA sequence encoding a ubiquitous product. The genetic system also contains multiple regulatory genes that control the action of structural genes.
However, many A or cistronic genes have multiple sites or sites where mutations can occur. A single nucleotide change can result in a mutated phenotype such as sickle cell anemia. Similarly, two defective ischemic strokes can recombine to form a wild-type cicerone. The functional concept remains the same as the concept of structural mutation and recombination properties of genes. This is the unit of inheritance. Exceptions (e.g.: duplicated genes, polyprotein genes, split genes, etc.) 2
Gene therapy
Gene therapy may be divided into two classes: germline gene remedy and somatic gene remedy. The distinction between the two strategies is that during somatic gene remedy, the. Genetic fabric is brought into unique target cells however the adjustments aren't surpassed on to the following generation, while in germline gene remedy, it's far processed or altered. when you consider that DNA changed into recognized because the fundamental unit of heredity, its potential to cause localized modifications in the human genome has made it a scientific goal. Gene therapy is defined because the ability to improve genes thru the alteration (mutation) of genes or site-specific adjustments for the reason of treatment. We also discuss various strategies usually used for this cause3 while many protocols are successful, gene therapy remains complicated and lots of techniques require further development. The unique cells that want remedy should be diagnosed and obtained. We need green methods to deliver genes to cells, and we want a better understanding of the disorder and its genetic interactions4
|
Disease |
Objective |
Stem cells |
Release mode |
Countries with the protocol |
|---|---|---|---|---|
|
Cystic fibrosis |
Enzymatic substitution |
Respiratory epithelium |
Adenovirus and liposome |
England and the United States
|
|
Familial hypercholesterolemia |
Substitution of low-density lipoprotein receptors |
Liver |
Retrovirus |
United States
|
|
Adenosine deaminase deficiency |
Substitution of the adenosine deaminase deficiency |
Blood |
Retrovirus |
Italy, Holland, and the United States
|
|
Fanconi anemia |
Complement C gene release |
Blood and bone marrow |
Retrovirus |
United States
|
|
α 1-antitrypsin deficiency |
Substitution of α 1-antitrypsin |
Respiratory epithelium |
Liposome |
United States
|
|
Rheumatoid arthritis |
Cytokine release |
Synovial membrane |
Retrovirus |
United States
|
|
Haemophilia B |
Factor IX substitution |
Skin fibroblasts |
Retrovirus |
China |
|
Gaucher Disease |
Glucocerebrosidase substitution |
Blood and bone marrow |
Retrovirus |
United States
|
Fig 1: Adapted from Misra S. Human gene therapy: a brief overview of the genetic revolution. J Assoc Physicians India. 2013;61(2):127-33. Review
Gene therapy processgene release
Review everyday gene are inserted into the genome to update unusual genes that reason positive diseases. some of the diverse challenges associated with this system, one of the primary ones are the issue of freeing genes into stem cells. consequently, molecular carriers known as 'vectors' are used to deliver the gene. This gene bought to be relatively particular, demonstrating the efficiency of releasing one or more genes inside the length required for the clinical application so as now not to be recognized through the immune machine. its miles are purified on a huge scale and in excessive concentrations, making it to be had for mass production and use. while the service enters the affected person, it cannot motivate an allergy or an inflammatory system. It has to enhance regular features, correct deficiencies, or prevent dangerous activities. in addition, it must be safe now not simplest for the patient but also for the surroundings and the professionals who manage it. subsequently, the carrier must be able to explicit the gene at some point in the patient's lifetime.3,5
The presence of viral genetic material inside the plasmid is a prime complicating issue because it could set off an acute immune response as well as capability tumorigenic transformation. There are presently major techniques for the genetic amendment of cells, mediated by the virus (desk 2) and through bodily mechanisms, from preparations acquired using advanced nanotechnologies.
Table 2 : Viral vectors for gene therapy
|
|
Retrovirus |
Lentivirus |
Herpes virus |
Adenovirus |
Adenoassociated |
Plasmid |
|---|---|---|---|---|---|---|
|
Provirus
|
RNA |
RNA |
RNA |
DNA |
DNA |
DNA |
|
Capacity
|
~9 kB |
~10kB |
>30kB |
~30kB |
~4.6kB |
Unlimited |
|
Adverse effects |
Insertional mutagenesis |
Insertional mutagenesis |
Inflammatory Response |
Inflammatory Response |
Mild Inflammatory Response |
No |
|
Integration Inti the recipient Genome
|
Yes |
Yes |
Yes |
No |
Extremely rare |
No |
|
Pre-existing immunity in the recipient
|
No |
No |
Yes |
Yes |
Yes |
No |
|
Duration of transgene expression
|
Long |
Long |
Transient |
Transient |
Long in postmitotic cells
|
Transient |
|
Germline transmission |
May occur |
yes |
No |
No |
May occur |
No |
Gene therapy for neonates with Angelman-Like Syndrome
Angelman Syndrome (AS) is an unprecedented (~1:15,000) neurodevelopmental disorder characterized by using excessive developmental put-off. intellectual incapacity, negative verbal exchange abilities, high occurrence of seizures, sleep disturbances, ataxia, Motor abnormalities, and microcephaly.6,7 AS is because of the lack of function of the UBE3A gene, inherited from the mother. UBE3A is placed on chromosome 15q11–thirteen and is expressed bivalently all through the body, however, best maternally.8,9 numerous genetic reasons result in AS including the new interstitial deletion of the maternal allele (~65-70% of the AS populace), loss-of-feature mutations A unilateral abnormality that takes place in maternal alleles (5-11%). in two paternal alleles with everyday function (3-7%) and diverse imprinting defects (3%).10,11 unmarried gene disorders are suitable candidates for precise gene and cellular remedy, and AS is particularly encouraging given the particular properties of UBE3A. The life of a silent but running reproduction of UBE3A Alleles of the ancestors enables the improvement of treatment options.Gene remedy is truly the switch of a therapeutic gene. treatment of any disorder or sickness. This therapy can be done ex vivo, with tissue harvested after which grown in the laboratory, or in vivo, wherein the patient is treated immediately frame. Precision remedy holds promise for lots of genes and illnesses in which traditional remedy has failed. There AS is resulting ina lack of functional UBE3A, a gene therapy designed to introduce a useful copy of UBE3A or antisense targeting, thereby silencing the native model most promising is the reactivation of the paternal allele The delivery is commonly intrathecal, requiring trendy anesthesia for Angelman infants. method of anesthesia has its dangers that have to be repeated 3-4 instances a year for human existence.12
Capabilities of UBE3A:
UBE3A encodes a hundred kDa ubiquitin-protein ligase. E3A changed into first defined as a conversation issue. between p53 and E6 oncoproteins in others the sort of papillomavirus to which E3 ubiquitin ligase binds its miles digested by p53 using the ubiquitin proteolysis system.13 This gene is ready at a hundred and twenty kB. while encoding a couple of isoforms which can be special Substrate specificity, multiple functions, and specific cell localization styles.14,15 UBE3A is a member of HECT (homologous to terminus E6-AP COOH) A family of enzymes that serve to transmit activated ubiquitin. Using the proteolytic machine, it breaks down into proteins and signals for degradation. many misunderstandings and unmarried amino acid insertion or deletion mutations located in AS affect the C-terminal catalytic domain. UBE3A additionally has a non-precise transcriptional function. free nuclear hormone receptor conjugate Ligase interest as a mutation affecting E6-AP activity Does not alter the co-activation ability, however, the function of UBE3A is protein ubiquitin ligase and transcription Stimulants are unambiguous, which results in loss of feature with precise etiology. In AS, maternal alleles are elusive. exciting, a couple of errors and single amino acid insertion or having effacing deletion mutations are seen in AS man or woman's C-terminal catalytic domain, hence the belief number one ligase feature is an imperative issue of the equipment. The is a sturdy correlation between lack of E6-AP ligase pastime and AS, as well as numerous protein targets worried in cellular proliferation and survival, synaptic feature, cell signaling, and worried device improvement recognized as UBE3A substrates UBE3A additionally serves as its substrate. in addition, UBE3A expression is related to the law of several genes worried in protein catabolism, cellular cycle, brain morphogenesis, and transcription law.
Imprint 15q11–13
Imprinting is an important epigenetic regulatory mechanism. because of maternal love, paternal alleles depend on the discernment of starting place and its miles a critical factor of AS. This process occurs within the germline wherein methyl companies are connected to DNA inside fragments. rich in cytosine guanine dinucleotide, the Formation of an imprinting area regulating expression within the q11–thirteen areas of chromosome 15 contains a cluster of imprinting genes, inclusive of UBE3A, which has maternal or paternal expression and is associated with numerous neurodevelopmental problems. Q11-13 There are two crucial imprinting areas: the Prader-Willi syndrome (PWS-IC) and the Angel imprinting center Syndrome effect Centre (AS-IC). PWS-IC is methylated at the maternal allele and represses the expression of genes upstream of UBE3A, consisting of MKRN3 (macron/ring finger protein 3), NDN (necdin), MAGEL2 (cancer-like antigen gene family member 2), and SNRPN (small ribonucleoprotein). polypeptide N), whilst PWS-IC is unmethylated at the paternal allele, allowing the expression of identical genes. AS-IC is turned into PWS-IC upstream. its miles are believed to assist through passing from father to mother Imprinting at some point of oogenesis and acts as a twin regulatory element with PWS-IC. genes downstream of UBE3A no longer contain GABRB3, GABRA5 and HERC2 imprinted and two alleles are expressed on both mothers 1536 N A. The reproduction unit starts on the PWS-IC and/or The SNRPN promoter terminates at the UBE3A promoter or ~40 kb outside the promoter. accurate How the ancestral UBE3A is silenced by using UBE3AATS is not understood. but, many mechanisms. Transcriptional interference has been proposed to be involved. RNA polymerase-related mechanisms of UBE3A-ATS Genes collide and disrupt transcription and RNA. Interference mechanism with the aid of which double-stranded RNA is formed among experience RNA and antisense RNA

Fig 3 : Mechanism of neuronal UBE3A imprinting and antisense oligonucleotide (ASO)-mediated unsilencing of paternal UBE3A gene expression. (a) overview of the UBE3A locus in neurons of a character with Angelman syndrome with a mutation inside the UBE3A gene (indicated with a celebrity). note that maximum sufferers with Angelman syndrome bring a maternally inherited deletion of the depicted region extending far past the Angelman syndrome Imprinting middle (AS-IC) and the UBE3A gene. (b) evaluatepaternal UBE3A expression within the Angelman syndrome circumstance upon ASO treatment (orange). Maternally imprinted genes are depicted in grey, and the AS-IC is indicated as an empty purple triangle. the dearth of a methylated Prader–Willi syndrome Imprinting middle (PWS-IC; indicated as an empty red circle) permits for the transcription of the lengthy non-coding SNHG14 gene, also referred to as UBE3A-ATS, which is liable for suppressing paternal UBE3A transcription (crimson rectangle). The administration of ASOs results in cleavage of the UBE3A-ATS transcript, ensuing in the unsilencing of the paternal UBE3A gene (depicted by a green rectangle), allowing recovery of the synthesis of the UBE3A protein.
Cystic fibrosis
Cystic fibrosis (CF) is a lifestyles-threatening autosomal-recessive disease because of mutations in an unmarried gene encoding cystic fibrosis transmembrane conductance regulator CFTR deficiency is a drug discovery goal and its improvements characteristic of CFTR results in ailment development those ensuing phenotypes have reignited interest and investment in different therapeutics focused on molecular, defects, consisting of gene remedy. The opposite Mutation elegance particular therapy as described above,Gene remedy is impartial of mutation magnificence and probably. appropriate for all sufferers. however, evidence of concept Gene therapy may additionally enhance cystic fibrosis lung sickness missing and UK consequences A non-viral section IIb look at has been finished by way of the CF Gene therapy Consortium. Lentiviral vectors display promise for cystic fibrosis Gene therapy due to their long-term expression and are powerful with repeated management. techniques based totally on zinc finger nucleases, transcription activator-like effector nucleases (TALENs), and extra lately CRISPR-Cas9 are generally utilized in vitro to generate in the context of the 16 CF in vivo version, Schwank and associates used CRISPR-Cas9 technology to The CFTR locus in human and mouse intestinal organoids. 17 in addition, Li and associates used zinc finger endonucleases Correction of the F508del mutation in an in vitro version18 However modification of the CFTR locus is currently underway. rather, it is more suitable for developing medical models A healing challenge within the subject of cystic fibrosis pulmonary remedy.
Gene Therapy to Treat CF Lung diseases
As noted above, cloning the CFTR gene becomes a milestone in improving gene remedies for cystic fibrosis. a lot of the paintings during the last decades have been used focusing on one development of gene remedies for cystic fibrosis lung sickness, the main motive is the urgent want for greater powerful treatments. Non-invasive lunge set admission to. The CFTR enhancer spec now licensed turned into Kalydeco (additionally called ivacaftor or VX-770) A achievement tale for the improvement of excessive-yield small-molecule pills. Kalydeco complements the feature of the CFTR protein sufferers with elegance III gating mutations 19,20 but, it is critical to observe that *only 4% of CF patients are providers of Mutations that respond to Cal decoGene therapy for CF has several crucial capabilities in comparison to different installed and investigational pills:
1. unlike the mutation elegance-specific remedies defined above, gene remedy is impartial to the mutation magnificence and is probably to be appropriate for all patients.
2. unlike many other known capsules, gene therapy is a molecular disorder at its supply. This offers the ability to prevent luailmentsent. start remedy earlythree. As stated earlier, the pathophysiology of the sickness is broadly debated. Lung gene theories no longer require entithe to amelioration of the disorder pathology. however, if CFTR expression has been proven to arise in inflammatory cells critical thing for the host to define extra gene remedy based on strategies consisting of bone marrow transfusion. The derived cells can be important
Viral vectors - intermediate gene transfer
First research: adenovirus vectors for gene therapy of cystic fibrosis
Adenoviruses are non-enveloped double-stranded nucleic acids Viruses encompass a complex icosahedral capsid.21,22 The nodule area of fibrils binds to coxsackievirus and adenovirus receptors (automobile) on the cellular surface and allows virus access. 21,23 enter via integrin receptors such as avb3 and avb5 the important histocompatibility complicated (MHC) became class they are also worried about viral cell interacti10 After getting into the virus continue to be within the nucleus in a transient state. because of the natural circuit of the lung and its huge
Packaging competencies and adenoviral vectors had been the first in their type assessment of gene remedy in cystic fibrosis. within the first-technology vector, The E1 location has been deleted to prevent viral replication. later Adjuvant-structured advanced, so-known asan adenoviral variation (HDAd) lacked all viral coding sequences. 24,25 the former, although now not placebo-managed, the gene was chased into by Zabner and colleagues in 1993, just four years after cloning the CFTR gene. serotype 2 Adenoviral (Ad2) vectors sporting human CFTR cDNA were Administered into the nasal epithelium of 3 patients with CF. even though the number of patients changed small, the observation nerve furnished the primary evil the hence of concept for chloride correction and sparked similar interest in growing adenovirus-primarily based medical trials.26 Taken together, these studies display that adenoviral vectors are not appropriate for CF mobile therapy because of (1) non-useful and brief gene transfer and (2) induction of immune responses which prevented overall performance after repeated management 21 the first AAV CF examination was posted in 1998. 6 exclusive medical trials focused on nasal transport of vectors through bronchoscopy or aerosol over eight years; Lungs or sinuses held out. these exams had been in particular performed. utilized by centered Genetics (now Amphiphile Biosciences) Serotype 2 (AAV2) 27 in the first single-dose section I take a look at, viral transport was to CF airlines sure, but I have had little chance to choose its efficiency A vector-particular CFTR expression. A massive red dose a look at (100 topics) effectiveto hit upon modifications in lung function did no longer attain its number one efficacy Endpoint (improved lung function)27 There are numerous reasons for this unhappiness Conclusions: (1) AAV2 is notably inefficient in airway transmission epithelial cells throughout the apical membrane; (2) vice versa Promoter Terminal Repeat (ITR) used for riding Expression of the four.7 kb CFTR cDNA became restricted The packaging ability (about 5 kb) of the virus has formerly been proven to assist CFTR expression whilst utilized. Slaughterhouse, however, may be very weak in one frame. and/or (3) reintroduction of AAV2 into the lung isn't always feasible. status quo of an antiviral immune reaction No similar pulmonary AAV research had been performed in view that 2005. but studies are being actively performed to deal with and enhance those potential boundaries of AAV.21
Are non-viral gene transfer agents suitable?
An alternative to viral vectors for CF gene therapy?
The non-viral gene transfer method includes (1) nucleic acid, i.e., healingcDNA and appropriate regulatory elements; and (2) a carrier molecule that binds to DNA. within the subject of gene remedy for cystic fibrosis, A huge quantity of provider molecules had been created that normally, can be called cationic lipids or Cationic polymers In vitro and in vivo preclinical airlines non-viral vector models are normally less green. Viral vector, however a direct evaluation in the human lung has now not been carried out.
1. THE interest OF THE '90S advanced A countless
Non-viral gene transfer agents, but we experience its development in the development of novel airway gene mixtures The treatment was modest and, only GL67A become used within the united kingdom's first non-viral lung look atabove) in 1999 It stays the best non-viral vector for the airway gene After more than a decade.28
2. slight and transient inflammatory reactions within the gene expression were then analyzed through Alton et al management of unmarried aerosols of GL67A/pCFTR within the lungs of patients with CF30 caused its development Plasmids without CpG nucleotides to obtain an artificial fabric Transcription regulator which includes elongation component 1a promoter and human cytomegalovirus (CMV) amplifier29 A plasmid lacking this new CpG infection by using warding off activation of Toll-like receptors long-time period (>4 weeks) gene expression within the (TLR)-nine pathway and mouse model.
Gene remedy Trialsthree.30 After selecting most strong non-viral gene switch agent (pGM169/GL67A), a segment I/IIa single-dose immunoassay became conducted to select the maximum suitable dose of pGM169/GL67A for the subsequent multidose immunoassay. This study projected an evaluation of Molecular efficacy (alternate in CFTR-based chloride delivery within the lungs and nostrils of dealt sufferers) (CFGTC manuscript underneath preparation
earlier than proceeding to a multiple-dose medical trial, toxicological research had been executed under the mounted policies. Mouse received 12 doses of pGM169/GL67A in 6 months sheep acquired nine monthly doses of pGM169/GL67 periods All animals tolerated the treatment properly. despite even though stent that the inflammatory reaction turned into discovered, Lung transformation or extrapulmonary response NOAEL (restrict of great facet consequences) is now not portedorted Multidose clinical trial ongoing.
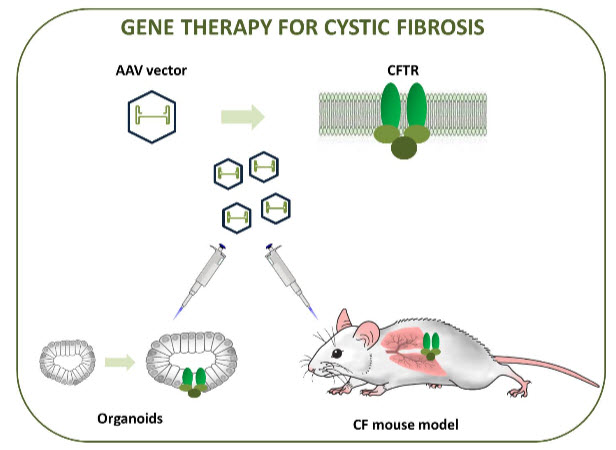
Fig 4: evaluation of the experimental design
FRAGILE X SYNDROME
Fragile X syndrome (FXS; OMIM #300624) is the maximum commonplace unmarried genetic cause of developmental cognitive impairment, with an anticipated prevalence of approximately 1:4,000 in men and 1:6,000 in females.33 FXS is resulting from a loss-of-feature mutation of the FMR1 gene positioned at Xq27.3, corresponding to the folate-sensitive locus FRAXA, which has been located cytogenetically in affected males.34This gene consists of 17 exons spanning 38 kb with CpG islands and volatile CGG repeats within the upstream promoter location 35,36 and encodes an RNA-binding protein. This triad consists of 5-55 repeats in a normal populace, enabling gene transcription and translation. inside this length variety, genes are passed on always across generations. when the CGG repeat is improved among fifty-six and two hundred (before initiation), the gene continues to transcribe (greater) mRNA, even though the initial repeat will become partially volatile.37 sooner or later, an extraordinary elegance of FMR1 alleles is the entire unmethylated mutation that has been reported rather in phenotypically normal adult males with >2 hundred CGG expansions that completely lack cytosine methylation (UFM).38,39 Characterization of cell traces derived from these people discovered that the FMR1 promoter DNA is unmethylated (in both the CpG growth and the FMR1 CpG island), transcription is increased (as in pre-mutation providers), FMRP ranges are approximately 30 is 40% concerning every different. beneath everyday situations (due to ribosome blockage in accelerated FMR1 mRNA 40
Transcriptional therapy for X frailty and other "epigenetic" disorders.
One of the most advanced clinical trials used glutamate metabolite receptor antagonists with promising results 41 DNA demethylation can be achieved with 5′-AzaC or more efficiently with 5′-aza-2′-deoxycytidine (5-AzadC), which is involved during cell replication as a deoxycytidine analog and is irreversible. DNA methyltransferase.42 The mechanism of regulation of FMR1 gene transcription reveals the complexity of the epigenetic events leading to heterochromatin formation, including DNA methylation, histone modification, and RNA interference, possibly secondary to interactions between FMR1 mRNA and its antisense transcript. Fragile X syndrome is therefore an informative model for studying potential ways to modulate chromatin activation, eventually leading to “transcriptional therapy”. Such treatment can be used for genetic disorders caused by DNA mutation (genetic) as well as disordered (epigenetic) expression of genes. Although acetylcarnitine does not reactivate the full FMR1 mutation, it significantly affects fragile-X patients, improving their adaptive and social behavior. We also tried valproic acid (VPA), as it was reported to increase histone acetylation. and demethylation of DNA, but again only minor reactivation appears to have been achieved. 43 Conversely, 5-azadC partially decreased histone H3 (H3-K4) lysine 9 methylation, while increasing histone acetylation and histone H3 (H3-K4) lysine 4 methylation. induced both. 44 It is associated with ALDPL1 and SMN2 45Adrenoleukodystrophy and Spinal Muscular Atrophy. Transcriptional activation can partially compensate for diseases that cause mutations in these two genes. Other genes with methylated CpG islands, such as fully mutated FMR1, also require DNA demethylation to resume transcription. We indeed showed that 5-azadC treatment is also effective in activating the FAM11A gene associated with the transcriptionally silenced Xq28 folate-sensitive vulnerable region in full-length FRAXF mutants.46 The same effect can be expected at other vulnerable sites by an expansion of the aforementioned CGG repeats and subsequent DNA methylation (FRAXE, FRA16A, etc.). The second obstacle is the need for cell division to be efficient; Interestingly, at least two reports suggest that 5-azadC may require little or no incorporation into DNA to effectively reduce the level of maintenance of DNA methyltransferase DNMT1. .47 However, a major objection to the use of drugs such as 5-azadC and HDAC inhibitors is that their effects are likely to be non-specific and genome-wideshowed that a very limited set of genes were indeed transcriptionally regulated by treatment with 5-AZC (51 genes), and/or trichostatin A (23 genes). The definitive treatment for "local" epigenetic disorders such as FXS or imprinting disorders may not rely on small molecules such as DNMT or HDAC inhibitors but on small RNAs designed to modulate silencing or targeted gene activation.
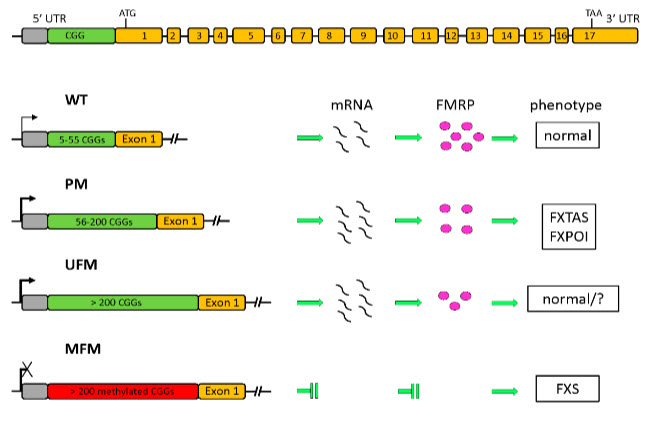
Fig 5 :Transcriptional Reactivation of the FMR1 Gene. a possible technique to the remedy of the delicate X Syndrome
CONCLUSION
The health of a neonate is very important following delivery and in the rest of the neonatal period as it may determine the subsequent health status of the baby throughout life. Therefore, after delivery, a careful examination of the baby should be carried out to determine signs or complications that need further assessment or management, primarily from the pediatrician or from other specialties, to ensure proper growth and development of the baby.
References
1. https://www.nature.com/articles/441398a
2. https://www.yourarticlelibrary.com/genetics/gene-types-and-functions-of-gene/12125
3. Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–910.
4. Misra S. Human gene therapy: a brief overview of the genetic revolution. J Assoc Physicians India. 2013;61(2):127–133. Review.
5. Gardlík R, Pálffy R, Hodosy J, Lukács J, Turna J, Celec P. Vectors and delivery systems in gene therapy. Med Sci Monit. 2005;11(4):RA110–RA121. Review.
6. Williams CA, Driscoll DJ, Dagli AI. Clinical and genetic aspects of Angelman syndrome. Genet Med. 2010;12(7):385-95.
7. Margolis SS, Sell GL, Zbinden MA, Bird LM. Angelman Syndrome. Neurotherapeutics. 2015;12(3):641-50.
8. Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15(1):70-3
9. Sutclife JS, Jiang YH, Galijaard RJ, Matsuura T, Fang P, Kubota T, et al. The E6-Ap ubiquitin-protein ligase (UBE3A) gene is localized within a narrowed Angelman syndrome critical region. Genome Res. 1997;7(4):368-77.
10. Fang P, Lev-Lehman E, Tsai TF, Matsuura T, Benton CS, Sutcliffe JS, et al. The spectrum of mutations in UBE3A causes Angelman syndrome. Hum Mol Genet. 1999;8(1):129-35
11. Jiang Y, Lev-Lehman E, Bressler J, Tsai TF, Beaudet AL. Genetics of Angelman syndrome. Am J Hum Genet. 1999;65(1):1-6
12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8608975/pdf/13311_2021_Article_1082.pdf
13. Huibregtse JM, Schefner M, Howley PM. A cellular protein mediates the association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10(13):4129-35.
14. Yamamoto Y, Huibregtse JM, Howley PM. The human E6-AP gene (UBE3A) encodes three potential protein isoforms generated by differential splicing. Genomics. 1997;41(2):263-6.
15. Dagli A, Buiting K, Williams CA. Molecular and Clinical Aspects of Angelman Syndrome. Mol Syndrome. 2012;2(3-5):100-12.
16. Cox DB, Platt RJ, and Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med 2015; 21:121–131.
17. Schwank G, et al. Functional repair of CFTR by CRISPR/ Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 2013;13:653–658.
18. Lee CM, Flynn R, Hollywood JA, et al. Correction of the DF508 mutation in the cystic fibrosis transmembrane conductance regulator gene by zinc-finger nuclease homology-directed repair. Biores Open Access 2012;1: 99–108.
19. Ramsey BW, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663–1672.
20. De Boeck K, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros 2014;13:674–680.
21. Volpert C, and Kochanek S. Adenoviral vectors for gene transfer and therapy. J Gene Med 2004;6 Suppl 1:S164–S171.
22. Saban SD, Silvestry M, Nemerow GR, et al. Visualization of a-helices in a 6-A˚ angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J Virol 2006;80:12049–12059.
23. Russell WC. Adenoviruses: update on structure and function. J Gen Virol 2009;90:1–20.
24. Griesenbach U, and Alton EW. Moving forward: cystic fibrosis gene therapy. Hum Mol Genet 2013;22:R52–R58.
25. Brunetti-Pierri N, and Ng P. Progress and prospects: gene therapy for genetic diseases with helper-dependent adenoviral vectors. Gene Ther 2008;15:553–560
26. Zabner J, Couture LA, Gregory RJ, et al. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 1993;75:207–216.
27. Moss RB, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther 2007;18:726–732.
28. McLachlan G, et al. pre-clinical evaluation of three nonviral gene transfer agents for cystic fibrosis after aerosol delivery to the ovine lung. Gene Ther 2011;18:996–1005.
29. Hyde SC, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol 2008;26:549–551.
30. Horsley AR, et al. Changes in cystic fibrosis lung disease's physiological, functional and structural markers with the treatment of a pulmonary exacerbation. Thorax 2013;68: 532–539.
31. Alton EW, et al. The safety profile of a cationic lipid-mediated cystic fibrosis gene transfer agent following repeated monthly aerosol administration to sheep. Biomaterials 2013;34:10267–10277.
32. Alton EW, et al. Toxicology study assessing efficacy and safety of repeated administration of lipid/DNA complexes to mouse lung. Gene Ther 2014;21:89–95.
33. Crawford DC, Meadows KL, Newman JL, Taft LF, Pettay DL, Gold LB, Hersey SJ, Hinkle EF, Stanfield ML, Holmgreen P, Yeargin-Allsopp M, Boyle C, Sherman SL. 1999. Prevalence and phenotype consequence of FRAXA and FRAXE alleles in a large, ethnically diverse, special education-needs population. Am J Hum Genet 64:495–507.
34. Lubs HA. 1969. A marker X chromosome. Am J Hum Genet 21:231–244.
35. Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu Y-H, Kuhl DPA, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang F, Eussen BE, van Ommen G-JB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST. 1991. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905–914.
36. Eichler EE, Richards S, Gibbs RA, Nelson DL. 1993. Fine structure of the human FMR1 gene. Hum Mol Genet 2:1147–1153.
37. Fernandez-Carvajal I, Lopez Posadas B, Pan R, Raske C, Hagerman PJ, Tassone F. 2009. Expansion of an FMR1 grey-zone allele to a full mutation in two generations. J Mol Diagn 11:306–310.
38. Smeets HJ, Smits AP, Verheij CE, Theelen JP, Willemsen R, van de Burgt I, Hoogeveen AT, Oosterwijk JC, Oostra BA. 1995. The normal phenotype in two brothers with a full FMR1 mutation. Hum Mol Genet 4:2103–2108.
39. Tabolacci E, Moscato U, Zalfa F, Bagni C, Chiurazzi P, Neri G. 2008a. Epigenetic analysis reveals a euchromatic configuration in the FMR1 unmethylated full mutations. Eur J Hum Genet 16:1487–1498.
40. Feng Y, Zhang F, Lokey LK, Chastain JL, Lakkis L, Eberhart D, Warren ST. 1995. Translational suppression by trinucleotide repeat expansion at FMR1. Science 268:731–734.
41. Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, Ramos FJ, Cornish K, He Y, Paulding C, Neri G, Chen F, Hadjikhani N, Martinet D, Meyer J, Beckmann JS, Delange K, Brun A, Bussy G, Gasparini F, Hilse T, Floesser A, Branson J, Bilbe G, Johns D, Gomez-Mancilla B. 2011. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with a differential response to the mGluR5 antagonist AFQ056. Sci Transl Med 3:64ra1.
42. Jackson-Grusby L, Laird PW, Magge SN, Moeller BJ, Jaenisch R. 1997. The mutagenicity of 5-aza-2*-deoxycytidine is mediated by the mammalian DNA methyltransferase. Proc Natl Acad Sci USA 94:4681–4685.
43. Chiurazzi P, Pomponi MG, Pietrobono R, Bakker CE, Neri G, Oostra BA. 1999. Synergistic effect of histone hyperacetylation and DNA demethylation in the reactivation of the FMR1 gene. Hum Mol Genet 8:2317–2323.
44. Tabolacci E, Pietrobono R, Moscato U, Oostra BA, Chiurazzi P, Neri G. 2005. Differential epigenetic modifications in the FMR1 gene of the fragile X syndrome after reactivating pharmacological treatments. Eur J Hum Genet 13:641–648.
45. Chang JG, Hsieh-Li HM, Jong YJ, Wang NM, Tsai CH, Li H. 2001. Treatment of spinal muscular atrophy by sodium butyrate. Proc Natl Acad Sci USA 98:9808–9813.
46. Shaw MA, Chiurazzi P, Romain DR, Neri G, Gécz J. 2002. A novel gene, FAM11A, associated with the FRAXF CpG island is transcriptionally silent in FRAXF full mutation. Eur J Hum Genet 10:767–772.
47. Ghoshal K, Datta J, Majumder S, Bai S, Kutay H, Motiwala T, Jacob ST. 2005. 5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, Bromo-adjacent homology domain, and nuclear localization signal. Mol Cell Biol 25:4727–4741.