{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Hanan. M. El Bsir1, Mokhtar. M. El-Baseir2, Abdulrhman .A . Akasha2*, Somaia.A . Elsaedi3 and Abdrheam Eluzy2.
1National Medical Research Center,
Zawia, Libya
2Department of Pharmaceutics,
Faculty of Pharmacy, University of Tripoli, Libya
3Minstry of Health,
Tripoli, Libya
ABSTRACT
This paper presents a survey study of generic and branded medicines available on the local market. The study was carried out on solid dosage forms (Tablets and capsules) for 53 medications available at the time of study in 69 pharmacies located in Tripoli, Libya. Physical specifications (generic name, batch number, manufacture date, expiry date and the manufacturer) as appeared on the package for each product were recorded in a designed form. The retail price at the time of study for each innovator brand and the counterpart generic was also recorded and compared. Results showed that 234 (89%) generics and 29 (11%) branded medicines were available for the studied medicines. The generics medicines were antibiotics (28%), cardiovascular (24.36%), analgesics and antipyretics (12.5%), gastrointestinal (12.8%), for respiratory tract (0.85%), anti-Anthistamin (7.26%), for adiabatic (3%), for vitamins and minerals (6%) and corticosteroid (1%) medicines. The original suppliers of the generics were European countries (42%), Arabian countries (41%) and other region of the world (17%). The retail prices for the generics were significantly (p > 0.05) lower compared to the price of innovator brand counterpart. The study suggests post-marketing surveillance studies for the available generics in comparison to the innovators especially in terms of therapeutic efficiency.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2617
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 10 Received On: 28/08/2018; Accepted On: 14/09/2018; Published On: 01/10/2018 How to cite this article: El Bsir, H., El-Baseir, M., Elsaedi, S., Akasha, A. and Eluzy, A. 2018. Generic Versus Branded Medicines Available on the Local Market. PharmaTutor. 6, 10 (Oct. 2018), 5-12. DOI:https://doi.org/10.29161/PT.v6.i10.2018.5 |
Methodology
This study involved a survey of generic and branded medicines available on the local privet market, Tripoli, Libya. The study was performed during the shift hours of 69 pharmacies located in different area of Tripoli. In order to avoid any adherence in data, no previous notes about the aim of the study was give to the pharmacies. Generic name, batch number, manufacture date, expiry date, strength and manufacturer name as appeared on the package for each medicament were recorded in predesigned forum. The local sale price for each drug at the time of study was also recorded and compared.
Data analysis
Data were analysed with Excel Version 2010.The number of generics for each dosage form (tablets and capsules) was recorded. The ratios of generics to innovators available for each drug were estimated. The price for the available innovator was compared to the lower sale price for the corresponding generic. The generics then classified according to their pharmacological group and compared. Two-sample t-test was used to test the significance of the difference in change in the measurement between the two groups was applied in comparison of data. The results of this study were expressed as % (95% Confidence Intervals (CI)). Variations were evaluated using the one-way analysis of variance (ANOVA) and
P≤0.05 was considered statistically significant.
Results
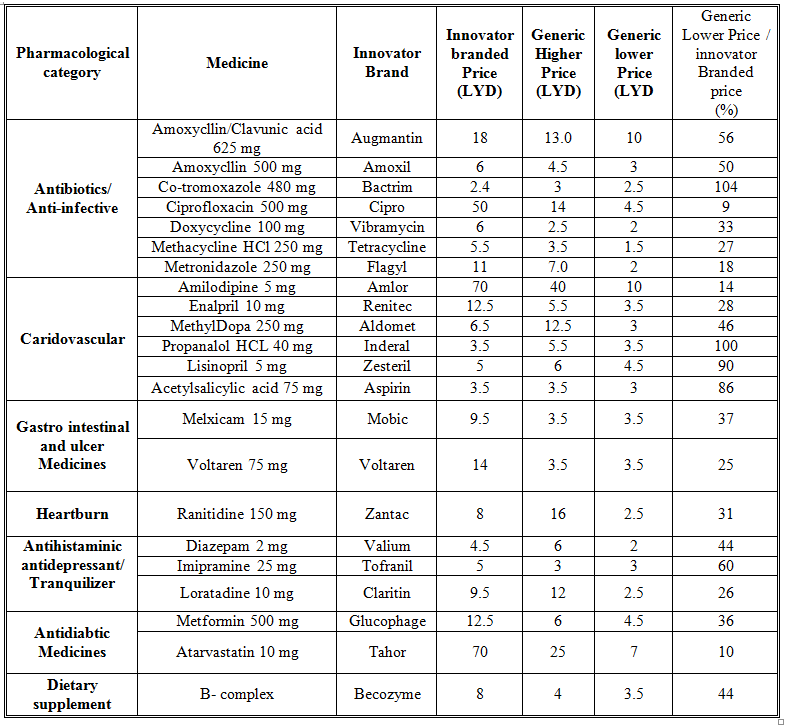
Overall, 53 medicines were surveyed in 69 pharmacies. The study revealed that 234 (89%) generics versus 29 (11%) innovators were available at the time of study for these medicines. Table 1 illustrates the generics and counterpart innovator brand for each medicine. The available generics were antibiotics (28%), analgesics and antipyretics (13%), gastrointestinal drugs (12%), cardiovascular systems (24%), respiratory tract infection treatment drugs (0.85%), anti-histamine (7%), anti-diabetic (2.5%), lipid lowering (5%), dietary supplement (6%) and corticosteroids (0.9%) drugs. Figure 1 shows the innovator brands (%) relative to the number of medicine for each pharmacological category. The highest innovator was for lipid lowering (22%) with lowest was for respiratory tract system with (0%) among other studied items. In contrast generics (%) were between 100 % for respiratory tract and 80% for Lipid lowering medicines respectively (Fig 2). Contrasting the total available generics for each pharmacological category to total number of generics showed that Antibiotic generics were with the highest percentage (28%) and respiratory and corticosteroids with the lowest (1%) available among other generics (Fig 3). Table 2 shows the local price for generics and their counterpart innovator brands for 23 medicines. Comparison study showed that 95% of generics were priced lower than their counterpart branded innovator. Two generics (5%) had similar prices to the innovator indicating that none of generics were priced more than the counterpart innovator. The prices of generics on an average were significantly lower than the innovator brands prices. In order to avoid any biased error due to different packaging systems, the prices of generics were also compared based on the unit price. Table 3 shows the unit prices for 21 medicines (generics and counterpart innovator brand) for different pharmacological categories. Results showed that the price of generic was always lower than the innovator price.
Table 1. Generic and innovator branded medicines available on the local market
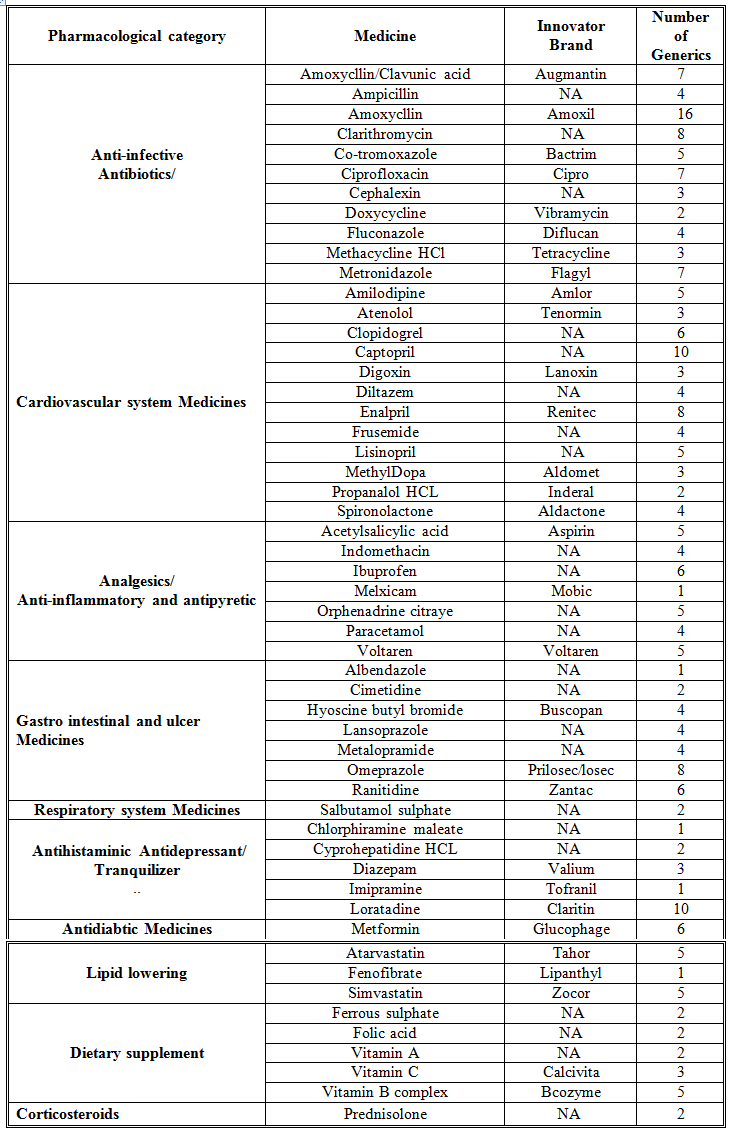
Table 2. Generic to Innovator Brand Price Differences
LYD = Libyan Dinar
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
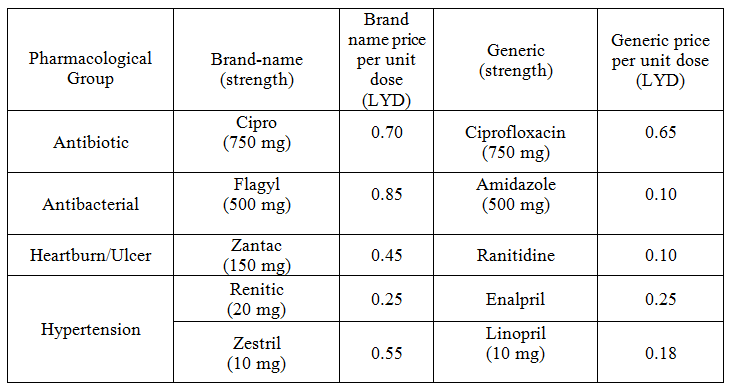
Table 3. Unit prices for branded name and contra part generic medicines

Figure 1. I innovator Brand (%) for the studied medicine of different Pharmacological category.
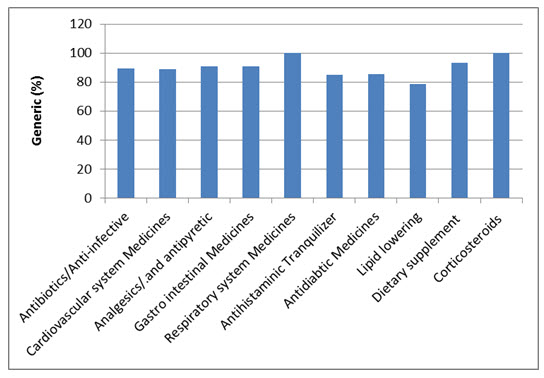
Figure 2. Generics (%) for the studied medicines of different pharmacological categories.
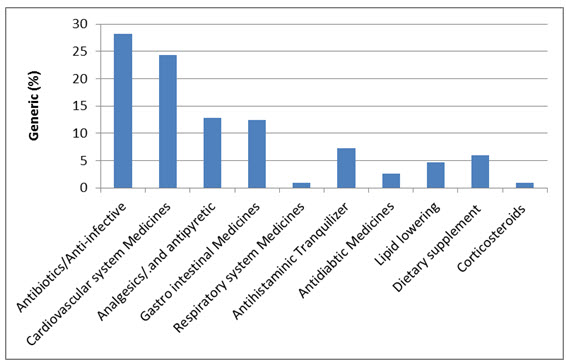
Figure 3. Pharmacological category generics (%) versus the total number of generics studied.
DISCUSSION
The use of generics in health service is controversial. Many patients and health care practitioners believe that brand name drugs are clinically superior to generic drugs. However, this opinion is maintained unfounded (Kesselheim, et al., 2008). Published summary of nearly 25 years of randomized clinical trials comparing brand name and generic cardiovascular medications addressed editorials concerning generic substitution for brand name drugs. Among all the drugs examined by the authors, no evidence indicated that brand name drugs were clinically superior to generic equivalents. Interestingly, though, of the 43 editorials written on generic substitution, more than half (53%) of the authors had a negative view of generic drug use. Our study showed similar trends as none of the pharmacological category drugs tested was free from generics. Several studies have been conducted to correlate the bioavailability of generic and branded antibiotics (Skipper and Vejlin, 2015), (Liu et al., 2009) & (Bagheri et al., 2014) . Post-market in-vitro bioequivalence study of six brands of ciprofloxacin tablets/caplets indicated that 3 of the 6 (50%) brands may not be used interchangeably with the chosen ‘innovator’ brand (Ngwuluka et al., 2009). The highest percentage (28%) of generics was for antibiotics that could be explained by the expired patient license for this group. Cardiovascular medicines were among the drugs studied in this work. The substitution of innovator cardiovascular medicines with generics still point of debate. Number of studies indicated the bioequivalence of generic cardiovascular drugs to the innovator but with no support for the substitution of branded drugs (Kesselheim et al., 2008). This study showed that the percentage of generic cardiovascular medicines available in the market was 24%. Some drugs are categorized as narrow therapeutic index where the margin of safety and toxicity is short. The small differences between therapeutic and toxic doses raised the concern over careful dosage adjustment and patient monitoring. Comparative bioavailability of three oral sustained release theophylline (narrow therapeutic index) with another brand in healthy human subjects showed no bioequivalent to reference product (Parvez et al., 2009). Our study did not cover this type of drug categories (antiepileptic) as these drugs are controlled and mostly dispensed through public sector in hospitals. Generics were supplied by many companies other than the innovator company. Pharmaceutical companies from industrialized countries, exporting generic drugs to emerging markets, can benefit from the favorably perceived country of origin (COO) of their drugs and thus help the acceptance of generic drugs in these markets (Smaoui et al., 2016). The countries of origin for the studied generics were Arabian countries (42%), European countries (41%) and the rest were countries from different regions. The cost of generics versus the innovator drug was shown to be a major factor in the acceptance of generics by physicians (Gossell-Williams, 2007). Generic drugs are typically less expensive than brand name drugs, and prices for generics have historically increased less than those for brand-name drugs (Kaiser, 2001). Patients purchasing innovator brands from private outlets usually pay substantially more than they would for generic equivalents (Dunne et al., 2013). Our study revealed similar findings. The prices of the available innovator drugs were found high compared to their generics counterpart for antibiotics, cardiovascular and lipid lowering drugs (Table 2). The unit price for innovator brand was also higher than the generic indication insignificant effect of the packaging and number of unites from different suppliers and manufactures (Table 3). Clearly these prices would have an impact on the affordability of the patient especially for long treatment drugs.
CONCLUSION
The outcome from this study suggests growing of generics versus branded medicines in respect of pharmacological category. Prices based on package and the unit was always lower for generic compared to their innovator counterpart. Post-marketing surveillance for the available generic in comparison to the innovator drugs especially for drugs with narrow therapeutic index is essential.
REFERENCES
1. Ameri, M.N.A., et al., (2012); "The differences between the branded and generic medicines using solid dosage forms: In-vitro dissolution testing"; Results in Pharma Sciences; 2; 1-8.
2. Antonio, P., et al.,(2004); "Bioequivalence study of two formulations of enalapril, at a single oral dose of 20 mg (tablets): A randomized, two-way, open-label, crossover study in healthy volunteers"; Current Therapeutic Research; 65(1) ; 34-46.
3. Bagheri H., Garraffo R., and Dellamonica P (2014); "From antiretroviral originator to generic drugs: Bioequivalence and pharmacovigilance"; Médecine et maladies infectieuses; 44(10); 464-469.
4. Dunne S., et al., (2013); "A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study"; BMC Pharmacology and Toxicology; 14(1); 1-19
5. Fischer, M. and Avorn J (2004);"Economic implications of evidence-based prescribing for hypertension: can better care cost less?" ; JAMA; 291(15); 1850–1856.
6. Fischer, M. and Avorn J (2004), " Potential savings from increased use of generic drugs in the elderly: what the experience of Medicaid and other insurance programs means for a Medicare drug benefit"; Pharmacoepidemiol Drug Saf; 13(4); 207–214.
7. Gossell-Williams, M., (2007); "Generic Substitutions: A 2005 Survey of the Acceptance and Perceptions of Physicians in Jamaica"; West Indian Med J; 56(5); 458.
8. Kaiser H.J., (2001); "Prescription Drug Trends—A Chart book Update"; Menlo Park, CA.
9. Kesselheim, A.S., et al., (2008); "Clinical Equivalence of Generic and Brand-Name Drugs Used in Cardiovascular Disease: A Systematic Review and Meta-analysis"; JAMA; 300 (21);2514–2526.
10. Kjoenniksen, I., Lindbaek M., and Granas A. G (2006); "Patients’ attitudes towards and experiences of generic drug substitution in Norway"; Pharm World Sci; 28(5); 284-289.
11. Kohl H., (2007); "Increasing generic drug use in Medicare Part D: The role of government"; J Am Geriatr Soc; 55(7);1106–1109.
12. Liu, Y.-M., Y.-H.K. Yang, and Hsieh C.R (2009); "Financial incentives and physicians’ prescription decisions on the choice between brand-name and generic drugs: Evidence from Taiwan"; Journal of Health Economics; 28(2) ; (341-349).
13. Meredith P., (2003) "Bioequivalence and Other Unresolved Issues in Generic Drug Substitution"; Clinical Therapeutics; 25(11); 2875-2890.
14. Minghetti, P., (1996); "Regulatory status of medicinal products for human beings in the European Union. The role of generic products"; Pharmacological research; 34(1); 3-7.
15. Ngwuluka N.C., Olorunfemi K.L., and Ochekpe N.A(2009); "Post-market in vitro bioequivalence study of six brands of ciprofloxacin tablets/caplets in Jos, Nigeria"; Scientific Research and Essay; 4(4); 298-305.
16. Parvez, N., et al., (2009) "Compartive bioavailability of three oral formulations of sustained release theophylline in healthy human subjects"; Indian J Pharmacol; 36(1); 29-33.
17. Shaw, S.J. and Hartman A.L,(2010); "The Controversy over Generic Antiepileptic Drugs"; J Pediatr Pharmacol Ther;. 15(2); 81-93.
18. Skipper, N. and Vejlin R, (2015); "Determinants of generic vs. brand drug choice: Evidence from population-wide Danish data"; Social Science and Medicine; 130; 204-215.
19. Smaoui, F., Kilani F.A, and Touzani M. (2016); "Country-of-origin versus brand: consumers’ dilemma when choosing between generic and branded drugs in emerging countries"; Journal of Product & Brand Management; 25(2); 148-159.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









