{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Sachin G. Dhandore*1, Kalyani B.Wagh2
1 Department of Pharmaceutics,
Shivnagar Vidya Prasarak Mandal’s College of Pharmacy,
Malegaon(Bk), Baramati, Pune Maharashtra, India
2 Jalna Education Society’s Institute of Pharmacy,
J.E.S. College Campus, Jalna Maharashtra, India
*sachindhandore5@gmail.com
ABSTRACT
Fluconazole is an imidazole derivative and used for the treatment of local and systemic fungal infection. The oral use of Fluconazole is not much recomanded as it has many side effects. Thus these formulations are made for better patient compliance and to reduce the dose of drug and to avoid the side effects like liver damage and kidney damage. The gel was formulated by using the synthetic polymer like carbapol 934 and HPMC by changing the polymer ratio. Gel formulations were characterized for drug content, pH determination, viscosity measurement, in-vitro diffusion. Among the six formulations F2 was selected as the best formulation as its %CDR after four and half hour was 99.01%. The viscosity of the F2 formulation was within the limit. Efficient delivery of drug to skin application was found to be highly beneficial in localizing the drug to desired site in the skin and reduced side effects associated with conventional treatment.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2578
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 4 Received On: 30/01/2018; Accepted On: 19/02/2018; Published On: 01/04/2018 How to cite this article: Dhandore SG, Wagh KB; Formulation and Evaluation of Fluconazole Gel by using Synthetic Polymer; PharmaTutor; 2018; 6(4); 27-31; http://dx.doi.org/10.29161/PT.v6.i4.2018.27 |
INTRODUCTION [Demuria et al.,1993, Agrawal et al.,Rashmi,2008, Loyd et al.]
Topical drug administration is localized drug delivery system anywhere in the body through ophthalmic, rectal, vaginal and skin as topical route. Skin is one of the most accessible organ of human body for topical administration and main route of topical drug delivery system. Number of medicated products is applied to the skin or mucous membrane that either enhances or restores a fundamental function of a skin or pharmacologically alters an action in the underlined tissues. Such products are referred as topical or dermatological products.
Fluconazole is an antifungal drug; Fluconazole fights opportunistic infections in people with HIV, several fungal infection. Fluconazole is antifungal agent of triazole class and it is new existing drug. It overcomes all side effects of the other fungal drugs like Ketoconazole, Amphotericin B, Clotrimazole and Miconazole. Even though it has some of side effects in the oral and IV dosage forms. Fluconazole remains one of the most frequent prescribed triazole because of its excellent bioavailability, tolerability and side effect profile. More than 80% of ingested drug is found in the circulation and 60 to 70 % is excreted in the urine. Only 10% of Fluconazole is protein bound.
Fluconazole also exhibits excellent tissue penetration.CSF levels are 70% of matched serum levels and levels reported in saliva, sputum and other sites are well within therapeutic ranges. The half-life is 27 to 34 hour in the presence of normal renal function allowing once daily dosing. In patients who have a reduced creatinine clearance the normal dose should be reduced by 50 %. Fluconazole serum levels are rarely necessary. Currently 50,100,150 and 200 mg tablets are available and IV formulation exits in 200 or 400 mg doses.
OBJECTIVES [Papps et al.,2004, Parashar et al.2013]
The present research investigation is planed with the following objectives;
1) To formulate gel of Fluconazole using synthetic polymer.
2) To evaluate the formulations with respect to various physical parameters.
3) To evaluate the formulations with respect to content uniformity, in-vitro diffusion studies.
MATERIALS AND METHODS
Materials
Fluconazole was procured from Cure Pharmaceuticals Limited Pune. Carbapol 934 was procured from Colorcon Asia Pvt. Ltd. and HPMC (Hydroxypropyl Methylcellulose), Alcohol, Methyl paraben, Propyl paraben were procured from Loba Chemicals Mumbai.
Methods [Modha et al.,2010, Shivhare et al.,2009]
UV Spectrum analysis of Fluconazole
The solution was scanned in the range of 200 to 400 nm to fix the maximum wavelength and UV spectrum was obtained.
Preparation of standard graph
Standard stock solution of Fluconazole
Accurately weighed 100mg of Fluconazole dissolved in 100ml of methanol from this stock solution 10ml was withdrawn and transferred into 100ml volumetric flask. Volume was made with methanol in order to get standard stock solution containing 100 μg/ml. From this standard stock solution a series of dilution (10,20,30,40,50 μg/ml) were prepared using methanol. The absorbance of these solutions was measured spectrophotometrically against blank of methanol at 260 nm.
PREPARATION OF GEL BASE [Patel et al.,2011, Golinkin,1979, Singh et al,2011]
The Fluconazole gel was prepared in 6 different batches that is F1 to F6 by changing the two polymers ratio carbapol 934 and HPMC while the other constant values were taken as mentioned in the formulae table 1. Carbapol 934 (2,3,4,5,6 % w/w) and purified water were taken in a beaker and allowed to soak for 24 hour. To this required amount of drug 2gm) was dispersed in water and then carbapol 934 was neutralized with sufficient quantity of NaOH. Glycerin as moistening agent, methyl paraben and propyl paraben as preservatives were added slowly with continuous gently stirring until the homogenous gel was formed
EVALUATION OF FLUCONAZOLE GEL [Karade et al.,2012, Bazigha et al.,2010]
Percentage yield
The empty container was weighed in which the gel formulation stored then again the container was weighed with gel formulation. Then substracted the empty container weighed with the container with gel formulation then it gives the practical yield. Then the percentage yield was calculated by the formula.
Percentage yield = (Practical yield/Theoretical yield) × 100
Drug content [Mitkari et al.,2010]
Weighed 10gm of each gel formulation were transferred in 250ml of volumetric flask containing 20ml of alcohol and stirred for 30 min. The volume was made up to 100ml and filtered. 1ml of above solution was further diluted to 10 ml with alcohol and again 1ml of the above solution was further diluted to 10ml with alcohol. The absorbance of the solution was measured spectrophotometrically at 260 nm. Drug content was calculated by following formula;
Drug content = (Absorbance/Slope) × Dilution factor × (1/1000)
Determination of pH [Denath et al.,2009,Prorost,1986]
Weighed 50 gm of each gel formulation were transferred in 10ml of beaker and measure it by using the digital pH meter. pH of the topical gel formulation should be between 3-9 to treat the skin infections.
Viscosity Estimation [Niyazbasha and Prakasam,2011]
The T-bar spindle (T95) was used for determining the viscosity of the gels. The factors like temperature, pressure and sample size etc which affect the viscosity was maintained during the process. The helipath T-bar spindle was moved up and down giving viscosities at number of points along the path.
The torque reading was always greater than 10 %. The average of three readings taken in one minute was noted as the viscosity of gels.
In-vitro diffusion study [Demuria et al.,1993]
The Abdominal skin of Albino mice weighing 20-25 gm of 8-10 week old was shaved using hand razor and clean the skin with hot water cotton swab. 5 gm of gel was applied uniformely to skin. The skin was mounted between the compartments of the Frantz diffusion cell with stratum corneum facing the donor compartments. Reservoir compartment was filled with 100ml phosphate buffer of pH 6.8.
The study was carried out at 37±10C and it carried out for 4 and 1//2 hour. 5 ml of sample was withdrawn from reservoir compartment at 30 minute interval and absorbance was measured spectrophotometrically at 260nm. Each time the reservoir compartment was replenished with the 5 ml of phosphate buffer pH 6.8 solutions to maintain constant volume.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS
Standard gragh of Fluconazole
The method for the estimation for the drug Fluconazole showed maximum absorption at wavelength 260 nm in alcohol and all obtained values given in the table 2. Standard curve obeyed Beer’s law at given concentration of 10 μg/ml and when subjected to regression analysis, the value of regression coefficient was found to be 0.997 which showed linear relationship between concentration and absorbance.
Percentage yield
The percentage yield of all formulation batches was calculated by using the following formula,
Percentage yield= (Practical yield/Theoretical yield) × 100
The minimum percentage yield was found in F1 formulation (96.45 %) and maximum percentage yield was found in the F6 formulation (99.01 %). The obtained values are tabulated in table 3.
Drug Content
After various formulation batches of Fluconazole gel the drug content of the formulated gel was estimated by UV Spectrtophotometer at wavelength 260 nm in alcohol. The results were given in the table 4. The minimum drug content was found in F4 batch (97.47 %) and maximum drug content found in the F2 batch (99.84 %).
pH Determination
The pH determination of all the formulation batches was done by using the digital pH meter (Global electronics, DPH 500).The obtained results are given in the table 5.The pH of all the formulation batches were found within the range that is 3-9.
Measurement of Viscosity
The measurement of all formulation batches of Fluconazole gel was measured by using the Brookfield Viscometer (PRO-II extra model, Brookfield viscometer, USA).The obtained values are tabulated in the table 6. The viscosity measured in centipoise (cps). F4 formulation batch was showed maximum viscosity (97548.11 cps).
IN-VITRO DIFFUSION STUDY [Gupta et al.,2010]
The release of Fluconazole from the gel was varied according to concentration of polymer. It has been concluded that, if we increase the concentration of polymer, the diffusion of drug through the skin also decreases. The amount of drug diffused from the formulation F2 was 99.01 in 4 ½ h which was higher among all the gel formulation. Resultant values obtained from this study are given in the table7.
Table 1: Formula for the gel
|
Ingredients |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Drug (gm) |
2 |
2 |
2 |
2 |
2 |
2 |
|
Alcohol (ml) |
4 |
4 |
4 |
4 |
4 |
4 |
|
Carbapol 934 (gm) |
2 |
4 |
6 |
8 |
- |
- |
|
HPMC (gm) |
- |
- |
- |
- |
4 |
5 |
|
Water (ml) |
60 |
60 |
60 |
60 |
60 |
60 |
|
Methyl paraben (gm) |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
|
Propyl paraben (gm) |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
Table 2: Calibration curve absorbance
|
Sr no. |
Concentration (mcg/ml) |
Absorbance at 260 nm |
|
1 |
0 |
0 |
|
2 |
10 |
0.260 |
|
3 |
20 |
0.446 |
|
4 |
30 |
0.633 |
|
5 |
40 |
0.823 |
|
6 |
50 |
0.999 |
Table 3: Percentage yield
|
Formulation Code |
Percentage yield (%) |
|
F1 |
96.45 |
|
F2 |
98.22 |
|
F3 |
99.41 |
|
F4 |
97.11 |
|
F5 |
98.22 |
|
F6 |
99.01 |
Table 4: Drug content
|
Formulation Code |
Drug content |
|
F1 |
97.54 |
|
F2 |
99.84 |
|
F3 |
98.65 |
|
F4 |
97.47 |
|
F5 |
99.29 |
|
F6 |
99.06 |
ssss
|
Formulation Code |
pH |
|
F1 |
6.9 |
|
F2 |
7.0 |
|
F3 |
7.1 |
|
F4 |
6.8 |
|
F5 |
6.9 |
|
F6 |
7.0 |
ssss
|
Formulation Code |
Viscosity (cps) |
|
F1 |
70,360.15 |
|
F2 |
83,481.74 |
|
F3 |
92,244.14 |
|
F4 |
97,548.11 |
|
F5 |
72,635.84 |
|
F6 |
82,746.87 |
Table 7 : In-vitro Diffusion study
|
Formulation code |
Time in min. |
||||||||||
|
0 |
30 |
60 |
90 |
120 |
150 |
180 |
210 |
240 |
270 |
||
|
% Drug Release |
F1 |
0 |
18.14 |
31.52 |
43.63 |
61.87 |
73.65 |
80.59 |
89.37 |
97.44 |
- |
|
F2 |
0 |
13.48 |
27.51 |
38.47 |
50.64 |
66.43 |
79.41 |
84.26 |
93.47 |
99.01 |
|
|
F3 |
0 |
13.15 |
26.17 |
39.61 |
49.22 |
63.49 |
76.91 |
82.33 |
90.46 |
94.66 |
|
|
F4 |
0 |
12.61 |
25.94 |
37.54 |
46.15 |
64.16 |
73.46 |
81.46 |
87.14 |
91.29 |
|
|
F5 |
0 |
14.99 |
27.16 |
40.14 |
55.34 |
69.39 |
83.97 |
90.63 |
94.19 |
98.93 |
|
|
F6 |
0 |
12.39 |
27.49 |
34.58 |
48.77 |
63.48 |
78.15 |
84.97 |
91.46 |
95.16 |
|
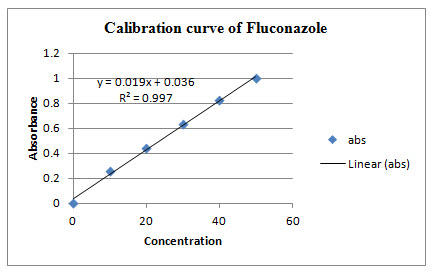
Graph 1: Calibration curve of Fluconazole
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
DISCUSSION
In the present study Fluconazole gel was prepared by using carbapol 934, HPMC, alcohol, methyl paraben, propyl paraben and distilled water. A total number of six formulations were prepared. The data obtained from percentage yield, drug content, viscosity studies, in-vitro diffusion studies skin gave satisfactory results.
CONCLUSION
1) Fluconazole is an imidazole derivative, used for the topical as well as systemic fungal infections. The bioavailability of Fluconazole is 90%. In the present study, an attempt was made to formulate topical gel of Fluconazole for efficient delivery of drug across the skin.
2) A suitable method of analysis of drug is UV spectrophotometry. Fluconazole showed maximum absorption at a wavelength of 260 nm in alcohol. The value of correlation coefficient was found to be r2 = 0.997, which showed linear relationship between concentrations and absorbance. Thus, it can be concluded that, beer’s law was obeyed.
3) Various formulations (F1, F2, F3, F4, F5 and F6) were developed by using suitable polymer (carbopol 934, HPMC) and penetration enhancer.
4) Developed formulation of Fluconazole were evaluated for the physicochemical parameters such as percentage yield, drug content, pH determination, viscosity, in-vitro diffusion.
5) From among all the developed formulations, F2 shows better drug diffusion for a period of 4 ½ h, did not produced skin irritation, good Rheological properties and good results of antifungal studies. Therefore, it was selected as the best formulation.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









