 About Authors:
About Authors:
Patel Chirag J*1, Prof. Satyanand Tyagi2, Patel Pinkesh1, Umesh Kumar1, Patel Jaimin1, Chaudhari Bharat1
1Department of Pharmaceutics, Maharishi Arvind Institute of Pharmacy, Mansarovar, Jaipur, Rajasthan, India-302020.
*Mr. Chirag Patel has published various Books, Research and Review articles. His academic works include 35 Publications (2 books was published in Lambert Academic Publishing, Germany & 8 Research Articles and 25 Review Articles was published in standard and reputed National and International Pharmacy journals)
2President & Founder, Tyagi Pharmacy Association (TPA) & Scientific Writer (Pharmacy), Chattarpur, New Delhi, India-110074.
*chirag.bangalore@gmail.com, +91-8000501871
ABSTRACT:
Transdermal drug delivery system is a type of convenient drug delivery system where drug goes to the systemic circulation through the protective barrier i.e. Skin. Skin acts as a major target as well as a principal barrier for topical / transdermal drug delivery. Vesicular system is one of the most controversial methods for transdermal delivery of active substances in that ethosome are the ethanolic phospholipids vesicles which are used mainly for transdermal delivery of drugs. Ethosomes have higher penetration rate through the skin as compared to liposomes hence these can be used widely in place of liposomes. The increased permeation of ethosomes is probably due to its ethanolic content. Ethanol increases the cell membrane lipid fluidity which results in increased skin penetrability of the ethosomes. These ethosomes permeates inside the skin and fuse with cell membrane lipids and release the drug. Hot and cold methods are used for formulation of ethosomes. Characterizations of ethosomesinclude Particle size, Zeta potential, Differential Scanning Calorimertry (DSC),Entrapment efficiency, Surface tension activity measurement, Vesicle stabilityand Penetration Studiesetc.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1571
INTRODUCTION
Transdermal drug delivery generated tremendous excitement and interest amongst major pharmaceutical companies in the 1980s and 90s. By the mid to late 1990s, the trend of transdermal drug delivery system merged into larger organizations. Over the year it has showed promising result in comparison to oral drug delivery system as it eliminates gastrointestinal interferences and first pass metabolism of the drug but the main drawback of TDDS is it encounters the barrier properties of the Stratum Corneum i.e. only the lipophilic drugs having molecular weight < 500 Da can pass through it [1].
Ethosomes are lipid vesicles containing phospholipids, alcohol (ethanol and isopropyl alcohol) in relatively high concentration and water.Ethosomes are soft vesicles made of phospholipids and ethanol (in higher quantity) and water. The size range of ethosomes may vary from tens of nanometers to microns (μ) [2]. ethosomes permeate through the skin layers more rapidly and possess significantly higher transdermal flux in comparison to conventional liposomes.Although, the exact mechanism for better permeation into deeper skin layers from ethosomes is still not clear. The synergistic effects of combination of phospholipids and high concentration of ethanol in vesicular formulations have been suggested to be responsible for deeper distribution and penetration in the skin lipid bilyers [2-5].
To improve the permeation of drugs through the skin various mechanisms have been investigated, including use of chemical or physical enhancers, such as iontophoresis, sonophoresis, etc. Liposomes, niosomes, transferosomes and ethosomes also have been reported to enhance permeability of drug through the stratum corneum barrier. Permeation enhancers increase the permeability of the skin, so that the drugs can cross through the skin easily. Unlike classic liposomes, [6] that are known mainly to deliver drugs to the outer layers of skin, ethosomes can enhance permeation through the stratum corneum barrier [7, 8].Ethosomes can entrap drug molecule with various physicochemical characteristics i.e. of hydrophilic, lipophilic, or amphiphilic [9, 10].
The ethosomes more advantages when compared to transdermal and dermal delivery. It delivers large molecules such as peptides, protein molecules. Simple method for drug delivery in comparison to Iontophoresis and Phonophoresis and other complicated methods. Low risk profile- The technology has no large-scale drug development risk since the tox-icological profiles of the ethosomal components are well documented in the scientific literature. High pa-tient compliance as it is administrated in semisolid form (gel or cream) and various application in Pharmaceutical, Veterinary, Cosmetic field [2].
[adsense:468x15:2204050025]
ADVANTAGES OF ETHOSOMAL DRUG DELIVERY
In comparison to other transdermal & dermal delivery systems
1. Enhanced permeation of drug through skin for transdermal drug delivery.
2. Delivery of large molecules (peptides, protein molecules] is possible.
3. It contains non-toxic raw material in formulation.
4. High patient compliance- The ethosomal drug is administrated in semisolid form (gel or cream) hence producing high patient compliance.
5. The Ethosomal system is passive, non-invasive and is available for immediate commercialization.
6. Ethosomal drug delivery system can be applied widely in Pharmaceutical, Veterinary, Cosmetic fields.
7. Simple method for drug delivery in comparison to Iontophoresis and Phonophoresis and other complicated methods [2].
ETHOSOMES COMPOSITION[1]
The Ethosomes are vesicular carrier comprise of hydroalcoholic or hydro/alcoholic/glycolic phospholipid in which the concentration of alcohols or their combination is relatively high. Typically, Ethosomes may contain phospholipids with various chemical structures like phosphatidylcholine (PC), hydrogenated PC, phosphatidic acid (PA), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylglycerol (PPG), phosphatidylinositol (PI), hydrogenated PC, alcohol (ethanol or isopropyl alcohol), water and propylene glycol (or other glycols). Such a composition enables delivery of high concentration of active ingredients through skin. Drug delivery can be modulated by altering alcohol: water or alcohol-polyol: water ratio. Some preferred phospholipids are soya phospholipids such as Phospholipon 90 (PL-90). It is usually employed in a range of 0.5-10% w/w. Cholesterol at concentrations ranging between 0.1-1% can also be added to the preparation. Examples of alcohols, which can be used, include ethanol and isopropyl alcohol. Among glycols, propylene glycol and Transcutol are generally used. In addition, non-ionic surfactants (PEG-alkyl ethers) can be combined with the phospholipids in these preparations. Cationic lipids like cocoamide, POE alkyl amines, dodecylamine, cetrimide etc. can be added too. The concentration of alcohol in the final product may range from 20 to 50%. The concentration of the non-aqueous phase (alcohol and glycol combination) may range between 22 to 70% (Table 1).
Table: 1 Different additive employed in formulation of Ethosomes [1]
|
Class |
Example |
Uses |
|
Phospholipid |
Soya phosphatidyl choline Egg phosphatidyl choline Dipalmityl phosphatidyl choline Distearyl phosphatidyl choline |
Vesicles forming component |
|
Polyglycol |
Propylene glycol Transcutol RTM |
As a skin penetration enhancer |
|
Alcohol |
Ethanol Isopropyl alcohol |
For providing the softness for vesicle membrane As a penetration enhancer |
|
Cholesterol |
Cholesterol |
For providing the stability to vesicle membrane |
|
Dye |
Rhodamine-123 Rhodamine red Fluorescene Isothiocynate (FITC) 6- Carboxy fluorescence |
For characterization study |
|
Vehicle |
Carbopol Ð934 |
As a gel former |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
INFLUENCE OF HIGH ALCOHOL CONTENT
Ethanol is an established efficient permeation enhancer [11] and is present in quite high concentration (20-50%) in Ethosomes. However, due to the interdigitation effect of ethanol on lipid bilayer, it was commonly believed that vesicles could not coexist with high concentration of ethanol [12]. Touitou [8] discovered and investigated lipid vesicular systems embodying ethanol in relatively high concentration and named them Ethosomes. The basic difference between liposomes and Ethosomes lies in their composition. The synergistic effect of combination of relatively high concentration of ethanol (20-50%) in vesicular form in Ethosomes was suggested to be the main reason for their better skin permeation ability. The high concentration of ethanol (20-50%) in Ethosomal formulation could disturb the skin lipid bilayer organization. Therefore, when integrated into a vesicle membrane, it could give an ability to the vesicles to penetrate the SC. Furthermore, due to high ethanol concentration the Ethosomal lipid membrane was packed less tightly than conventional vesicles but possessed equivalent stability. This allowed a softer and malleable structure giving more freedom and stability to its membrane, which could squeeze through small openings created in the disturbed SC lipids [13]. In addition, the vesicular nature of Ethosomal formulations could be modified by varying the ratio of components and chemical structure of the phospholipids. The versatility of Ethosomes for systemic delivery is evident from the reports of enhanced delivery of quite a few drugs like acyclovir, minoxidil, triphexyphenidyl, testosterone, cannabidol and zidovudine.
METHODS OF PREPARATION ETHOSOMES
Ethosomes can be prepared by two very simple and convenient methods that is hot method (Fig. 1) and cold method (Fig. 2)
1. Cold Method:
This is the most common method utilized for the preparation of ethosomal formulation. In this method phospholipid, drug and other lipid materials are dissolved in ethanol in a covered vessel at room temperature by vigorous stirring with the use of mixer. Propylene glycol or other polyol is added during stirring. This mixture is heated to 300?C in a water bath. The water heated to 300?C in a separate vessel is added to the mixture, which is then stirred for 5 min in a covered vessel. The vesicle size of ethosomal formulation can be decreased to desire extend using sonication or extrusion method. Finally, the formulation is stored under refrigeration [14, 15].
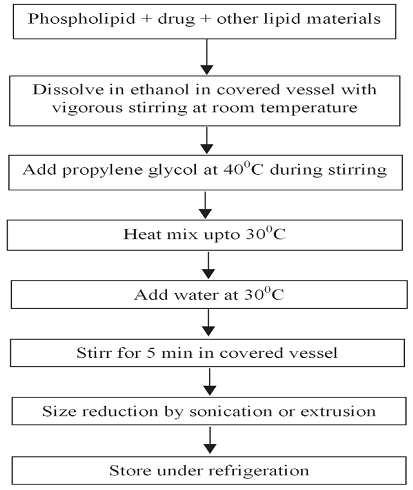
Figure 1: Cold method for the preparation of ethosomes [14]
2. Hot method
In this method phospholipid is dispersed in water by heating in a water bath at 400C until a colloidal solution is obtained. In a separate vessel ethanol and propylene glycol are mixed and heated to 400?C. Once both mixtures reach 400?C, the organic phase is added to the aqueous one. The drug is dissolved in water or ethanol depending on its hydrophilic/ hydrophobic properties32, 31. The vesicle size of ethosomal formulation can be decreased to the desire extent using probe sonication or extrusion method [14, 15].
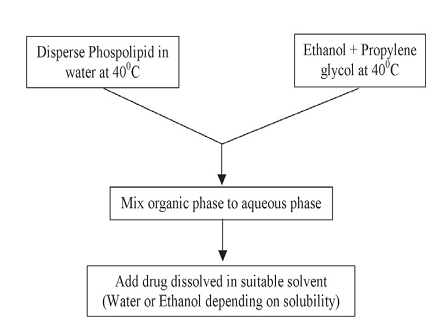
Figure 2: Hot method for the preparation of ethosomes [14]
CHARACTERIZATIONS OF ETHOSOMES
1. Visualization
Visualization of ethosomes can be done using transmission electron microscopy (TEM) and by scanning electron microscopy (SEM) [14].
2. Vesicle size and Zeta potential
Particle size and zeta potential can be determined by dynamic light scattering (DLS) using a computerized inspection system and photon correlation spectroscopy (PCS) [16].
3. Entrapment Efficiency
The entrapment efficiency of drug by ethosomes can be measured by the ultra centrifugation technique [17].
4. Differential scanning calorimertry (DSC)
Transition temperature (Tm) of the vesicular lipid systems was determined by using the Mettler DSC 60 computerized with Mettler Toledo star software system (Mettler, Switzerland).The transition temperature was measured by using the aluminium crucibles at a heating rate 10 degree/minute, within a temperature range from 20?C–300?C [17, 18].
5. Surface Tension Activity Measurement
The surface tension activity of drug in aqueous solution can be measured by the ring method in a Du Nouy ring tensiometer [17, 18].
6. Vesicle Stability
The stability of vesicles can be determined by assessing the size and structure of the vesicles over time. Mean size is measured by DLS and structure changes are observed by TEM [14, 17, 18].
7. Penetration and Permeation Studies
Depth of penetration from ethosomes can be visualized by confocal laser scanning [14].
EVALUATION TESTS
1. Filter Membrane-Vesicle Interaction Study by Scanning Electron Microscopy 40
Vesicle suspension (0.2 mL) was applied to filter membrane having a pore size of 50 nm and placed in diffusion cells. The upper side of the filter was exposed to the air, whereas the lower side was in contact with PBS (phosphate buffer saline solution), (pH 6.5). The filters were removed after 1 hour and prepared for SEM studies by fixation at 4°C in Karnovsky’s fixative overnight followed by dehydration with graded ethanol solutions (30%, 50%, 70%, 90%, 95%, and 100% vol/vol in water). Finally, filters were coated with gold and examined in SEM (Leica, Bensheim, Germany) [1, 14, 19].
2. Skin Permeation Studies
The hair of test animals (rats) were carefully trimmed short (<2 mm) with a pair of scissors, and the abdominal skin was separated from the underlying connective tissue with a scalpel. The excised skin was placed on aluminium foil, and the dermal side of the skin was gently teased off for any adhering fat and/or subcutaneous tissue. The effective permeation area of the diffusion cell and receptor cell volume was 1.0 cm2 and 10 mL, respectively. The temperature was maintained at 32°C ± 1°C. The receptor compartment contained PBS (10 mL of pH 6.5). Excised skin was mounted between the donor and the receptor compartment. Ethosomal formulation (1.0 mL) was applied to the epidermal surface of skin. Samples (0.5 mL) were withdrawn through the sampling port of the diffusion cell at 1-, 2-, 4-, 8-, 12-, 16-, 20-, and 24-hour time intervals and analyzed by high performance liquid chromatography (HPLC) assay [1, 14].
3. Stability Study
Stability of the vesicles was determined by storing the vesicles at 4°C ± 0.5°C. Vesicle size, zeta potential, and entrapment efficiency of the vesicles was measured after 180 days using the method described earlier [1].
4. Vesicle-Skin Interaction Study by TEM and SEM
From animals ultra thin sections were cut (Ultracut, Vienna, Austria), collected on formvar-coated grids and examined under transmission electron microscope. For SEM analysis, the sections of skin after dehydration were mounted on stubs using an adhesive tape and were coated with gold palladium alloy using a fine coat ion sputter coater. The sections were examined under scanning electron microscope [14, 19].
5. Vesicle-Skin Interaction Study by Fluorescence Microscopy
Fluorescence microscopy was carried according to the protocol used for TEM and SEM study. Paraffin blocks are used, were made, 5-μm thick sections were cut using microtome (Erma optical works, Tokyo, Japan) and examined under a fluorescence micro Cytotoxicity Assay MT-2 cells (T-lymphoid cell lines) were propagated in Dulbecco's modified Eagle medium (HIMEDIA, Mumbai, India) containing 10% fetal calf serum, 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mmol/L Lglutamine at 37°C under a 5% CO2 atmosphere. Cytotoxicity was expressed as the cytotoxic dose 50 (CD50) that induced a 50% reduction of absorbance at 540 nm [1, 2, 14].
6. Drug Uptake Studies
The uptake of drug into MT-2 cells (1×106 cells/mL) was performed in 24-well plates (Corning Inc) in which 100 μL RPMI medium was added. Cells were incubated with 100 μL of the drug solution in PBS (pH 7.4), ethosomal formulation, or marketed formulation, and then drug uptake was determined by analyzing the drug content by HPLC assay [1, 2, 14, 19].
7. HPLC Assay
The amount of drug permeated in the receptor compartment during in vitro skin permeation experiments and in MT-2 cell was determined by HPLC assay using methanol: distilled-water :acetonitrile (70:20:10 vol/vol) mixture as mobile phase delivered at 1 mL/min by LC 10-AT vp pump (Shimadzu, Kyoto, Japan). A twenty-microliter injection was eluted in C-18 column (4.6×150 mm, Luna, 54, Shimadzu) at room temperature. The column eluent was monitored at 271 nm using SPDM10A vp diode array UV detector. The coefficient of variance (CV) for standard curve ranged from 1.0% to 2.3%, and the squared correlation coefficient was 0.9968 [1, 14, 19].
8. Statistical Analysis
Statistical significance of all the data generated was tested by employing ANOVA followed by studentized range test. A confidence limit of P < 0.05 was fixed for interpretation of the results using the software PRISM (GraphPad, Version 2.01, San Diego, CA) [1, 2].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
APPLICATIONS OF ETHOSOMES
1. Transdermal Delivery of Hormones
Oral administration of hormones is associated with problems like high first pass metabolism, low oral bioavailability and several dose dependent side effects. The risk of failure of treatment is known to increase with each pill missed [20].
Touitou et al. compared the skin permeation potential of testosterone Ethosomes (Testosome) across rabbit pinna skin with marketed transdermal patch of testosterone (Testoderm patch, Alza). They observed nearly 30-times higher skin permeation of testosterone from ethosomal formulation as compared to that marketed formulation.
2. Delivery of anti-parkinsonism agent
Dayan and Touitou prepared ethosomal formulation of psychoactive drug trihexyphenidyl hydrochloride (THP) and compared its delivery with that from classical liposomal formulation. THP is a M1 muscarinic receptors antagonist and used in the treatment of Parkinson disease. The results indicated better skin permeation potential of ethosomal-THP formulation and its use for better management of Parkinson disease [1].
3. Pilosebaceous Targeting
Hair follicles and sebaceous glands are increasingly being recognized as potentially significant elements in the percutaneous drug delivery. Furthermore, considerable attention has also been focused on exploiting the follicles as transport shunts for systemic drug delivery [21]. With the purpose of pilosebaceous targeting, Maiden et al. prepared and evaluated minoxidil ethosomal formulation.
4. Topical Delivery of DNA
Many environmental pathogens attempt to enter the body through the skin. Skin therefore, has evolved into an excellent protective barrier, which is also immunologically active and able to express the gene [22]. On the basis of above facts another important application of ethosomes is to use them for topical delivery of DNA molecules to express genes in skin cells. Touitou et al. in their study encapsulated the GFP-CMV-driven transfecting construct into ethosomal formulation. They applied this formulation to the dorsal skin of 5-week male CD-1 nude mice for 48 hr. After 48 hr, treated skin was removed and penetration of green fluorescent protein (GFP) formulation was observed by CLSM. It was observed that topically applied ethosomes-GFP-CMV-driven transfecting construct enabled efficient delivery and expression of genes in skin cells. It was suggested that ethosomes could be used as carriers for gene therapy applications that require transient expression of genes. These results also showed the possibility of using ethosomes for effective transdermal immunization. Gupta et al. recently reported immunization potential using transfersomal formulation. Hence, better skin permeation ability of ethosomes opens the possibility of using these dosage forms for delivery of immunizing agents [1].
5. Transcellular Delivery
Touitou et al. in their study demonstrated better intracellular uptake of bacitracin, DNA and erythromycin using CLSM and FACS techniques in different cell lines. Better cellular uptake of anti-HIV drug zidovudine and lamivudine in MT-2 cell line from ethosomes as compared to the marketed formulation suggested ethosomes to be an attractive clinical alternative for anti-HIV therapy [3, 4].
6. Delivery of Anti-Arthritis Drug
Topical delivery of anti-arthritis drug is a better option for its site-specific delivery and overcomes the problem associated with conventional oral therapy. Cannabidol (CBD) is a recently developed drug candidate for treating rheumatoid arthritis. Lodzki et al. prepared CBD-ethosomal formulation for transdermal delivery. Results shows significantly increased in biological anti-inflammatory activity of CBD-ethosomal formulation was observed when tested by carrageenan induced rat paw edema model. It was concluded encapsulation of CBD in ethosomes significantly increased its skin permeation, accumulation and hence it’s biological activity [1].
7. Delivery of Antibiotics
Topical delivery of antibiotics is a better choice for increasing the therapeutic efficacy of these agents. Conventional oral therapy causes several allergic reactions along with several side effects. Conventional external preparations possess low permeability to deep skin layers and subdermal tissues [23]. Ethosomes can circumvent this problem by delivering sufficient quantity of antibiotic into deeper layers of skin. Ethosomes penetrate rapidly through the epidermis and bring appreciable amount of drugs into the deeper layer of skin and suppress infection at their root. With this purpose in mind Godin and Touitou prepared bacitracin and erythromycin loaded ethosomal formulation for dermal and intracellular delivery. The results of this study showed that the ethosomal formulation of antibiotic could be highly efficient and would overcome the problems associated with conventional therapy.
8. Delivery of Problematic drug molecules
The oral delivery of large biogenic molecules such as peptides or proteins is difficult because they are completely degraded in the GI tract. Non-invasive delivery of proteins is a better option for overcoming the problems associated with oral delivery [24]. Dkeidek and Touitou investigated the effect of ethosomal insulin delivery in lowering blood glucose levels (BGL) in vivo in normal and diabetic SDI rats. In this study a Hill Top patch containing insulin ethosomes was applied on the abdominal area of an overnight fated rat. The result showed that insulin delivered from this patch produced a significant decrease (up to 60%) in BGL in both normal and diabetic rats. On the other hand, insulin application from a control formulation was not able to reduce the BGL.
Verma and Fahr [25] reported the cyclosporin A ethosomal formulation for the treatment of inflammatory skin disease like psoriasis, atopic dermatitis and disease of hair follicle like alopecia areata etc. Paolino et al. [26] investigated the potential application of ethosomes for dermal delivery of ammonium glycyrrhizinate. Ammonium glycyrrhizinate is naturally occurring triterpenes obtained from Glycyrrhizinate Glabra and useful for the treatment of various inflammatory based skin diseases [27].
9. Delivery of Anti-Viral Drugs
Zidovudine is a potent antiviral agent acting on acquired immunodeficiency virus. Oral administration of zidovudine is associated with strong side effects. Therefore, an adequate zero order delivery of zidovudine is desired to maintain expected anti-AIDS effect [28]. Jain et al. [5] concluded that ethosomes could increase the transdermal flux, prolong the release and present an attractive route for sustained delivery of zidovudine.
Acyclovir is another anti-viral drug that widely used topically for treatment of Herpes labialis [29].The conventional marketed acyclovir external formulation is associated with poor skin penetration of hydrophilic acyclovir to dermal layer resulting in weak therapeutic efficiency. It is reported that the replication of virus takes place at the basal dermis. To overcome the problem associated with conventional topical preparation of acyclovir [30]. Horwitz et al. formulated the acyclovir ethosomal formulation for dermal delivery. The results showed that shorter healing time and higher percentage of abortive lesions were observed when acyclovir was loaded into ethosomes.
DISCUSSION AND CONCLUSION
Ethosomes have become an area of research interest, because of its enhanced skin permeation, improved drug delivery, increased drug entrapment efficiency etc. Ethosomes are the non invasive drug delivery carriers that enable drugs to reach the deep skin layers finally delivering to the systemic circulation. Enhanced delivery of bioactive molecules through the skin and cellular membranes by means of an ethosomal carrier opens numerous challenges and opportunities for the research and future development of novel improved therapies. Ethosomes provides a number of important benefits including improving the drug's efficacy, enhancing patient compliance and comfort and reducing the total cost of treatment. The Ethosomes were found to be suitable for various applications within the pharmaceutical, biotechnology, veterinary, cosmetic, and nutraceutical markets.
REFERENCES
1. Kumar KP, Radhika PR, Sivakumar T, “Ethosomes-A Priority in Transdermal Drug Delivery”, International Journal of Advances in Pharmaceutical Sciences, 2010, 1, 111-121.
2. Gangwar S, Singh S, Garg G, “Ethosomes: A novel tool for drug delivery through the skin”, Journal of Pharmacy Research 2010, 3 (4), 688-691.
3. Touitou E, Godin B, Dayan N, Weiss C, Piliponsky A, Levi-Schaffer F, “Intracellular delivery mediated by an ethosomal carrier”, Biomaterials, 2001, 22, 3053-3059.
4. Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M, “Ethosomes novel vesicular carriers for enhanced delivery: characterization and skin penetration properties”, J Control Release, 2000, 65, 403-418.
5. Jain S, Umamaheshwari RB, Bhadra D, Jain NK, “Ethosomes: a novel vesicular carrier for enhanced transdermal delivery of an anti-HIV agent”, Ind J Pharma Sci, 2004, 66, 72-81.
6. Heeremans JLM, Gerristen HR, Meusen SP, Mijnheer FW, Gangaram RS, Panday G, Prevost R, Kluft C, Crommelin DJA, “The preparation of tissue type plasminogen activator (t- PA) containing liposomes: entrapment efficacy and ultracentrifugation damage”, J Drug Target, 1995, 3, 301.
7. Asbill CS, El-Kattan AF, Michniak B, “Enhancement of transdermal drug delivery: chemical and physical approaches”, Crit Rev Therapeut Drug Carrier Sys, 2000, 17, 621.
8. Touitou E, Dayan N, Levi-Schaffer F, Piliponsky A, “Novel lipid vesicular system for enhanced delivery”, J Lip Res, 1998, 8, 113.
9. Bhalaria MK, Naik S, Misra AN, “Ethosomes: A novel delivery system for antifungal drugs in the treatment of topical fungal diseases”, Indian Journal of Experimental Biology 2009, 47, 368-375.
10. Verma DD, Fahr A, “Synergistic penetration effect of ethanol and phospholipids on the topical delivery of Cyclosporin A”, J. Control Release, 2004, 97, 55-66.
11. Berner B, Liu P, “Alcohol, In Percutaneous Enhancer, Smith EW, Maibach HI, Ed.; CRC Press, Boca Raton, FI, 1995, 45-60.
12. Riaz M, Weiner N, Martin F, Liberman HA, Reiger MM, Banker GS, Ed.; Marcel Dekker, New-York, Basel, In Pharmaceutical Dosage forms, Disperse Systems, 1998, 2, 567-600.
13. Barry BW, “Novel mechanisms and devices to enable successful transdermal drug delivery”, Eur. J. Pharm. Sci., 2001, 14, 101-114.
14. Nikalje AP, Tiwari S, “Ethosomes: A Novel Tool for Transdermal Drug Delivery”, IJRPS, 2012, 2 (1), 1-20.
15. Dinesh D, Amit AR, Maria S, Awaroop R L, Mohd HGD, “Drug vehicle based approaches of penetration enhancement”, Int. J. Pharm. Pharm. Sci., 2009, 1 (1), 24-45.
16. Maghraby GM, Williams AC, Barry BW, “Oestradiol skin delivery from ultra deformable liposomes: refinement of surfactant concentration” Int. J. Pharma., 2000, 63-74.
17. Fry DW, White JC, Goldman ID, “Rapid secretion of low molecular weight solutes 1from liposomes without dilution”, Analytical Biochemistry 1978, 90, 809-815.
18. Cevc G, Schatzlein A, Blume G, “Transdermal drug carriers: Basic properties, optimization and transfer efficiency in case of epicutaneously applied peptides”, J. Cont. Release, 1995, 36, 3-16.
19. Lopez-Pinto JM, Gonzalez-Rodriguez ML, Rabasco AM, “Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes”, Int. J. Pharma., 2005, 298, 1-12.
20. Johnsen SG, Bennett EP, Jensen, VG Lance, “Therapeutic effectiveness of oral testosterone” 1974, 2, 1473-1475.
21. Lauer AC, Ramachandran C, Lieb L.M, Niemiec S, Weiner ND, “Targeted delivery to the pilosebaceous unit via liposomes” Adv. Drug Delivery, 1996, 18, 311-324.
22. Tuting TH, Storkus WJ, Falo J, “DNA Immunization Targeting the Skin: Molecular Control of Adaptive Immunity”, J. Invest. Dermatol., 1998, 111, 183-188.
23. Fang J, Hong C, Chiu W, Wang Y, “Effect of liposomes and niosomes on skin permeation of enoxacin”, Int. J. Pharm., 2001, 219, 61-72.
24. Chetty DJ, Chien YW, “Transdermal Delivery of CaCO3-Nanoparticles Containing Insulin”, Crit Rev Ther Drug Carrier Syst.,1998, 15, 629-670.
25. Verma DD, Fahr A, “Synergistic penetration effect of ethanol and phospholipids on the topical delivery of Cyclosporin A”, J. Control Release., 2004, 97, 55-66.
26. Paolino D, Lucania G, Mardente D, Alhaique F, Fresta M, “Innovative Drug Delivery Systems for the Administration of Natural Compounds”, J. Control. Release., 2005, 106, 99-110.
27. Fu Y, Hsieh J, Guo J, Kunicki J, Lee MY, Darzynkiewicz Z, Wu J.M, Licochalcone A, “Anti-inflammatory efficacy of Licochalcone A: correlation of clinical potency and in vitro effects”, Biochem. Biophys. Res. Commun., 2004, 322, 263-270.
28. Kim S, Chien YW, “Toxicity of cationic lipids and cationic polymers in gene delivery”, J. Control. Release., 1996, 40, 67-76.
29. Spruance SL, Semin, “The natural history of recurrent oral facial herpes simplex virus infec tion”, Dermatol., 1992, 11, 200-206.
30. Fiddan AP, Yeo JM, Strubbings R, Dean D, “Vesicular Approach for Drug Delivery into or Across the Skin”, Br. Med. J., 1983, 286, 1699.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









