About Authors: Akanksha Patel1, S. P. Pawar2
1. M.Pharm, sem-II, Department of Pharmaceutics, A. R. College of pharmacy, V. V. Nagar - 388120, Anand Gujarat, India
2. Associate Professor, Department of Pharmaceutics, A. R. College of pharmacy, V. V. Nagar - 388120, Anand, Gujarat, India
Reference ID: PHARMATUTOR-ART-1074
Abstract
Recent years development in nanotechnology has opened up new horizons in the field of drug and gene delivery. Gold nanoparticles are one of the most versatile platform of nanotechnology. Here the emerging applications of them are discussed. Various approaches like direct drug conjugates, surface modification and light stimulus to achieve desired goals are utilized. Most promising applications of gold nanoparticles is organ targeting. Liver targeting, brain targeting and tumour targeting is achieved precisely with gold nanoparticles. Magnetic targeting in combination with iron nanoparticles can also be achieved with gold nanoparticles. Photodynamic and photothermal therapies for the destruction of tumours is possible with gold nanoparticles due to their unique property, localized surface plasmon resonance (LSPR). Gene delivery is the forthcoming application of gold nanoparticles. DNA can be made available to the site in intact form. Gold Nanoparticles are also used for the management diabetes. Gold nanoparticles are a good carrier for insulin. Gold nanoparticles are popular for the delivery of anticancer agents. New applications are still emerging for delivery of anticancer agents with gold nanoparticles. Due to their unique optical characteristics they are also used in the field of diagnostics. They have therefore been proposed as contrast agents for bioimaging, or as heating devices. Acoustic signals obtained are then utilized. This imaging technique allows recognition of early biochemical, physiological, and anatomical changes before manifestation of gross pathological changes.
[adsense:336x280:8701650588]
Introduction
Recent years’ advancement in nanoparticles drug delivery is expected to change the scenario of pharmaceutical and biotechnology industries. [1] Gold nanoparticles (AuNPs) has appeared as an attractive candidate for delivery of various drug molecules or large biomolecules like protein, DNA, RNA to their targets. [2]
Gold nanostructures have proven to be a versatile platform for a broad range of biomedical applications, with potential use in numerous areas including: diagnostics and sensing, in vitro and in vivo imaging and therapeutic techniques. [3] These applications are possible because of the highly favorable properties of gold nanoparticles, many of which can be modified for specific applications. Gold nanoparticles (AuNPs) are promising nanocarriers for biomedical applications due to their tractable synthesis, ease of functionalization, biocompatibility, and non-toxicity. The unique chemical and physical properties of gold nanoparticles (AuNPs) provide versatility in delivery method and tunability of surface properties. [4], [5]
Although many reviews have been published on gold nanoparticles, here we narrow the focus to consider only emerging applications of gold nanoparticles in field of drug and gene delivery as well as diagnostics.
Approaches
Various approaches can be adapted to utilize gold nanoparticles for desired applications. They are discussed briefly in following section:
1. Direct Drug Conjugates
Gold nanoparticles can be directly conjugated to drug molecules by physical absorption or via ionic or covalent bonding. Conjugates of gold nanoparticles with antibiotics provide promising results in the treatment of intracellular infections. [6] In general, the amount of antibiotics used in therapy is much higher than the actual dose required for pathogenic destruction. The excess amount of antibiotics can produce adverse effects. [7] Therefore, the conjugation of gold nanoparticles with antibiotics for targeted drug delivery would be a possible way to improve antibiotic efficacy.
Gold nanoparticles can be directly conjugated with antibiotics or other drug molecules by physical absorption or via ionic or covalent bonding. Different antibiotics were directly conjugated to gold nanoparticles of about 14 nm diameter. The gold nanoparticles were conjugated to ampicillin, streptomycin and kanamycin by physical interaction. Significant reduction in the Minimum Inhibitory concentration (MIC) was noted in case of streptomycin and kanamycin and slight decrement in MIC of ampicillin was observed. [6]
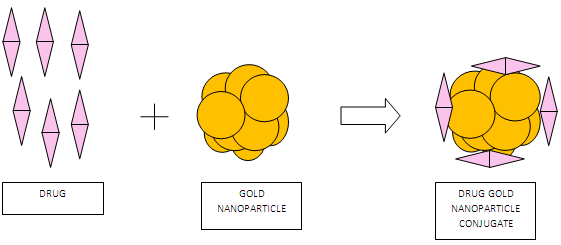
Fig 1: Schematic representation of direct drug conjugates by physical interaction
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
The electrostatic conjugation of drug to gold nanoparticles was also explained. Doxorubicin was conjugated to gold via amino group. The reaction proceeded under mild acidic conditions. Gold nanoparticles could not be adsorbed on doxorubicin in alkaline solution because amino group was not protonated. However, under acidic conditions, protonation created a positively charged amino group thus adsorption was feasible.
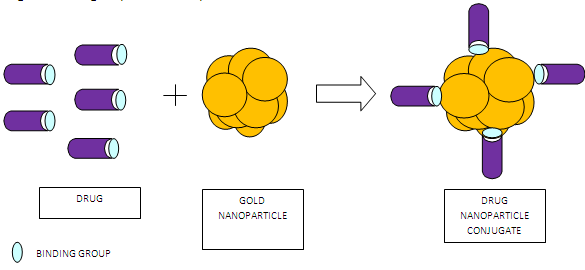
Fig 2: Schematic representation of direct drug conjugates by electrostatic interaction
2. Surface Modification
The main drawback of gold nanoparticles is their tendency to be recognized by the complement system and subsequently elimination by macrophages. In fact, almost 90% of intravenously administered nanoparticles are taken up by liver and spleen within several minutes and are thus not available for localized activity in the target tissue. The efficiency of elimination is based on the particle size and surface properties such as charge or hydrophobicity. In order to obtain particles that are less recognized and eliminated, particles should possess hydrophilic and uncharged surfaces. For the development of particles that stay in the circulation for predetermined intervals for drug targeting, it is necessary to closely examine the surface properties of the particles and modify them for the desired application. It is therefore crucial to modify the nanoparticles surface in order to achieve desired characteristics. [5]
For surface modification various polymers are used. They alter the charge and hydrophobicity of the gold nanoparticles and provide desired characteristics. Eg. : Poly Ethyleneglycol, Polyepsiloncaprolactone
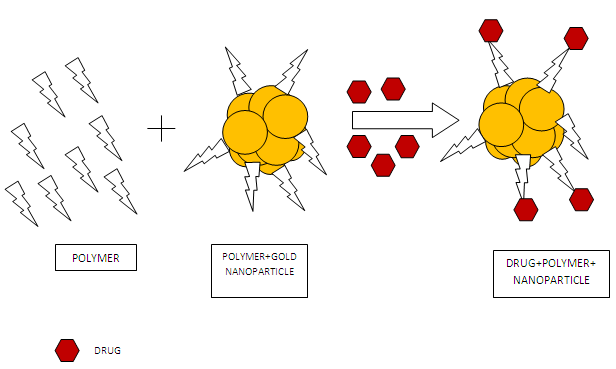
Fig 3: Schematic representation of surface modification
3. Using Light as an External Stimulus
The gold nanoparticles possess interesting optical properties. The colloidal solution is either an intense red color (for particles less than 100 nm) or a dirty yellowish color (for larger particles) differing with bulk gold due to their unique interaction with light. In the presence of the oscillating electromagnetic field of the light, the free electrons of the metal nanoparticles undergo an oscillation with respect to the metal lattice. This process is resonant at a particular wavelength of the light and is termed the Localized Surface Plasmon Resonance (LSPR). Gold nanospheres with particle size around 10 nm in diameter have a strong absorption maximum around 520 nm in aqueous solution due to their LSPR. [9] After absorption, the surface plasmon decays radiatively resulting in light scattering or nonradiatively by converting the absorbed light into heat. [10] They have therefore been proposed as contrast agents for bioimaging, or as heating devices for photothermal therapy. [11]
Emerging Application of Gold Nanopaarticles
Emerging applications of gold nanoparticles are described below:
• For liver targeted drug delivery
Nitric oxide (NO) plays an important role in inhibiting the progress of hepatic fibrosis and as a result complication of portal hypertension by inhibiting human hepatic stellate cell (HSC) activation. Gold nanoparticles based drug delivery system containing NO donors was developed which was of potential therapeutic application in chronic liver disease. HSC proliferation and the vascular tube formation ability, manifestations of their activation, were significantly weakened by the NO released from these nanoparticles. This study indicates that gold nanoparticles mediated drug delivery systems for introducing NO could be used as a strategy for the treatment of hepatic fibrosis or chronic liver diseases. [12]
• For brain targeted drug delivery
Gold nanoparticles have promising utilities for drug delivery as well as for the diagnosis and treatment of several diseases related to the CNS. AuNPs are retained in a number of organs, such as liver and spleen due to their negative charge and/or processes of Opsonization and hence decreasing their delivery to the brain. It is crucial to alter the nanoparticles’ surface to obtain desired characteristics for achieving enhanced delivery to the brain. conjugation of 12 nm GNPs with the amphipathic peptide CLPFFD increases the in vivo penetration of these particles to the rat brain. The C(GNP)-LPFFD did not alter the integrity of the blood-brain barrier, and had no effect on cell viability. [5]
• For magnetic drug targeting
Magnetic drug targeting, using core-shell magnetic carrier particles loaded with anti-cancer drugs, is an emerging and significant method of cancer treatment. Kayal et al. synthesized Gold shell-iron core nanoparticles (Fe@Au) by the reverse micelle method with aqueous reactants, surfactant, co-surfactant and oil phase. From Magnetic measurements it was found that the particles were superparamagnetic at room temperature. The anti-cancer drug doxorubicin was loaded onto these Fe@Au nanoparticle carriers. It was found that the amine (-NH2) group of DOX binds to the gold shell. The present studies show that DOX loaded gold coated iron nanoparticles are promising for magnetically targeted drug delivery. [13]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
• For Photodynamic therapy (PDT)
Photodynamic therapy (PDT) is a promising technique for treating various cancers. It involves light, photosensitizers, and tissue oxygen. Most photosensitizing agents, such as porphyrins and are hydrophobic and locate preferentially in apolar sites such as the lipid bilayer membranes of cells. After intravenous injection and accumulation in the target tissue, the photosensitizers, can be excited by light of an appropriate wavelength, can transfer energy to surrounding tissue oxygen which leads to generation of highly reactive oxygen species (e.g., singlet oxygen), and induce apoptosis or necrosis directly. [1]
On one hand, such PDT drugs need to be lipophillic to get through the lipophillic membranes to reach to relevant sites so that the initial oxidative damage can occur on proteins that reside within the membranes of those organelles. But lack of solubility under physiological conditions raises a significant problem for intravenous PDT drug delivery. The administration of such photosensitizers takes long time to reach the maximum accumulation in the tumor sites. This poses an additional risk for toxicity and side effects.
Therefore, one needs a delivery carrier that can bestow hydrophilicity during the drug delivery without destroying the hydrophobic characteristics of the drug itself. There are several delivery strategies known to stabilize PDT drugs in aqueous systems. Conjugated polymer nanoparticles are one of them.
PEGylated gold nanoparticles (AuNPs) hold the promise to be a highly efficient PDT drug delivery scaffold. Au NPs are well known for their chemical inertness and have minimum toxicity. By using water-soluble polyethylene glycol (PEG), NPs can be stabilized by steric repulsion to inhibit colloid aggregation in physiological conditions. Drugs on the NPs could be shielded from being uptaken by the reticuloendothelial system (RES). Such drug vectors can preferentially accumulate in tumor sites through the leaky tumor neovasculature and do not return to the circulation. [1], [14]
• As insulin carrier
Gold nanoparticles synthesized with chitosan were tested as a carrier for insulin. The nanoparticles showed good insulin loading and long-term stability in terms of aggregation. The use of chitosan served dual purpose by acting as a reducing agent in the synthesis of gold nanoparticles and also promoting the penetration and uptake of insulin across the oral and nasal mucosa in diabetic rats. The study concluded that oral and nasal administration of insulin-loaded, chitosan-reduced gold nanoparticles improved pharmacodynamic activity of insulin. [15]
• For delivery of anti HIV agents
Gold nanoparticles coated with multiple copies of an amphiphilic sulfate-ended ligand are able to bind the HIV glycoprotein gp120 as measured by surface plasmon resonance (SPR) and inhibit in vitro the HIV infection of T-cells. It is possible to attach both sulfated ligands and other anti-HIV molecules on the same gold cluster and so one can design multifunctional anti-HIV systems. [16]
• For delivery of anticancer agents
The anticancer drug, doxorubicin (DOX), was loaded onto DNA-capped gold nanoparticles (AuNPs) designed for specific DOX intercalation. [17]
To improve selectivity and antitumour activity functionalization of gold nanoparticles (AuNPs) with both a targeting peptide and a drug peptide ligand was attempted. Enhanced activity and selectivity of the peptide multifunctionalized gold nanoparticle conjugates was observed. [18]
Gold nanoparticles (AuNPs) were functionalized with an anticancer drug, doxorubicin. As discussed earlier, Doxorubicin was assembled on gold via amino group. The reaction proceeded under mild acidic conditions. Au NPs could not be adsorbed on doxorubicin in alkaline solution because amino group was not protonated. Under acidic conditions, protonation created a positively charged amino group thus adsorption was easier. The interaction between Au colloids and doxorubicin is believed to be electrostatic. [19]
Multidrug resistance (MDR) is a major hurdle to the success of cancer chemotherapy. Doxorubicin was tethered onto the surface of gold nanoparticles with a poly (ethylene glycol) spacer via an acid-labile linkage (DOX-Hyd@AuNPs), it was demonstrated that multidrug resistance in cancer cells can be significantly overcome by a combination of efficient cellular entry and a responsive intracellular release of doxorubicin from the gold nanoparticles in acidic organelles. It released doxorubicin in response to the pH of acidic organelles following endocytosis [20]
Methotrexate is a folic acid analogue which destroys folate metabolism of cells and is commonly used as a cytotoxic anticancer drug. The carboxylic groups on the methotrexate molecule can bind to the surface of gold nanoparticles. The cytotoxic effect of methotrexate conjugated to goldnanoparticles in Lewis lung carcinoma cells is about seven times higher than that of free methotrexate. [21]
• For gene delivery
The use of nucleic acids to treat and control diseases is termed ‘gene therapy’. Gold nanoparticle conjugates can prepared which protect DNA from degradation. [4]
The light-triggered release of deoxyribonucleic acid (DNA) from gold nanoparticle-based, plasmon resonant vectors, such as nanoshells, shows great promise for gene delivery in living cells. Intracellular light-triggered release can be performed on molecules that associate with the DNA in a DNA host-guest complex bound to nanoshells. The light exposure required for molecular release do not compromise cell viability. This highly controlled co-release of nonbiological molecules accompanying the oligonucleotides may have vast applications in the study of cellular processes and in the development of intracellular targeted therapies. [22]
• For percutaneous drug delivery
Co administration of protein drugs with gold nanoparticles enables percutaneous delivery of them. The Au-NPs with a mean size of 5 nm are skin permeable, probably due to the nano-bio interaction with skin lipids and the transient and reversible openings on the stratum corneum. When simultaneously applied with Au-NPs, the protein drugs were also granted to penetrate the skin barrier and migrate into the deep layers. This indicated that co-administration with the skin-permeable Au-NPs could mediate proteins across the skin barrier. Such co-delivery effect can lead to a simple yet effective method for overcoming the skin barrier for percutaneous protein drug delivery. Employing this strategy, a non-invasive vaccine delivery system was developed and by topically co-administrating antigens with Au-NPs immune response was elicited in the tested animals. The results conferred the promise for achieving a needleless transcutaneous vaccination. [23]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
• For photothermal therapy
As discussed earlier, Gold nanorods (which are rod-shaped gold nanoparticles) show a surface plasmon band in the near-infrared region. They have been proposed as heating devices for photothermal therapy. Polyethylene glycol-modified gold nanorods systemically administrated. After intravenous injection of gold nanorods followed by near-infrared laser irradiation, significant tumor damage triggered by the photothermal effect was observed. [24]
For diagnosis
Due to its unique properties such as surface-plasmon and photothermal effects, gold nanoparticles have emerged as an effective diagnostic tool.
Computed tomography (CT) is one of the most useful diagnostic tools among commonly used biomedical imaging techniques, but currently available CT contrast agents i.e. small iodinated molecules, possess a number of limitations, including a lack of targeted molecular imaging, short imaging time, and renal toxicity. By functionalizing the surface of gold nanoparticles (GNPs) with a prostate-specific membrane antigen (PSMA) RNA aptamer that binds to PSMA, a targeted molecular CT imaging system capable of specific imaging of prostate cancer cells that express the PSMA protein was obtained. Intensity was 4 times more than with the monoiodinated molecules. [24]
Monoclonal antibodies (mAbs) can recognize a specific cancer cell. When such monoclonal antibodies attached to gold nanoparticles or nanorods and then expose it to near IR radiation "heating phenomenon" results which can be used in cancer detection. This acoustic signal gives valuable information about the presence of cancer cells. [25]
Advantages of this technique are
1. Gold nanoparticles are not toxic to human cells. A similar technique with QDs uses semiconductor crystals to mark cancer cells, but the semiconductor material is potentially toxic to the cells and humans.
2. It does not require expensive high-powered microscopes or lasers to view the results. All it takes is a simple, inexpensive microscope and white light.
3. The results are instantaneous. If a cancerous tissue is sprayed with gold nanoparticles containing the antibody, the results can be seen immediately. The scattering is so strong that a single particle can be detected [25]
Conclusion
Gold nanoparticles are promising new vehicle for drug and gene delivery. Control, place and timing of release of the candidate is vital in drug and gene delivery systems. The poor stability of conventional drugs and genes in biological fluids, enzymatic degradation, and difficulties in assuring their penetration through some barrier or nucleus of cells are some of the unfavorable attributes of the existing technologies. The conjugation of gold nanoparticles with drugs or genes offers greater control and enhanced therapeutic efficacy. In particular, the combination of gold nanoparticles and laser irradiation to control the release of drugs and genes has the potential to provide useful therapeutic benefits. Nevertheless, it is early days in this field and one can achieve a lot.
References
1. Prajapati B.G. Nanoparticles As Platforms For Targeted Drug Delivery System In Cancer Therapy. The Internet Journal of Nanotechnology [ISSN: 1937-8262],2009 May [cited on 2011 April 11]; 3910:[about 1 p.] Available from: https:/www.ispub.com/2009/3/ nanoparticles as platforms
2. Murthy RSR, Vyas SP, Narang RK, Nanocolloidal carriers site specific and controlled drug delivery, first edition, CBS publishers and distributors, New Delhi, 2011, p.197
3.Cobley CM, Chen J, Cho EC, Wang LV, Xia Y, Gold nanostructures: a class of multifunctional materials for biomedical applications, Chem Soc Rev. 2011 Jan;40(1):44-56
4.Pissuwan D., Niidome T., Cortie M.B., The forthcoming applications of gold nanoparticles in drug and gene delivery system, Journal of controlled release, 149(2011), 65-71
5. Kim C., Ghosh P. and Rotello V.M, Multimodal drug delivery using gold nanoparticles, Nanoscale, 2009, 1, 61-67
6. Saha B., Bhattacharya J., Mukherjee A., Ghosh A.K., Santra C.R., Dasgupta A.K. et al, In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics, Nanoscale Res. Lett. 2 (2007) 614–622
7. Rang H.P., Dale M.M., Ritter J.M., Moore P.K., Pharmacology, , first edition Churchill livingstone,Elsevier science limited,2003,p.629
8. Mirza AZ, Shamshad H, Preparation and characterization of doxorubicin functionalized gold nanoparticles, Eur J Med Chem., 2011 May; 46(5):1857-6
9. Mody V. V., Siwale R., Singh A., Mody H. R., Introduction to metallic nanoparticles, J Pharm Bioallied Sci, 2010 Oct–Dec; 2(4): 282–289.
10. Jain PK, Huang X, El-Sayed IH, El-Sayed MA, Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008 Dec;41(12):1578-86
11. Niidome T, Shiotani A, Akiyama Y, Ohga A, Nose K, Pissuwan D, Niidome Y, Theragnostic approaches using gold nanorods and near infrared light, Yakugaku Zasshi, 2010 Dec;130(12):1671-7
12. Das A., Mukherjee P., Singla S.K., Guturu P.,. Frost M. C, Mukhopadhyay D., Shah V. H. and Patra C., Fabrication and characterization of an inorganic gold and silica nanoparticle mediated drug delivery system for nitric oxide, Nanotechnology,2010,vol.3, no.30
13. Kayal S, Ramanujan RV, Anti-cancer drug loaded iron-gold core-shell nanoparticles (Fe@Au) for magnetic drug targeting, J Nanosci Nanotechnol, 2010 Sep;10(9):5527-39
14.Cheng Y., Sámi A.C., Meyers J.D.,Panagopoulos I., Fei B., and Burda C. Highly Efficient Drug Delivery with Gold Nanoparticle Vectors for in Vivo Photodynamic Therapy of Cancer, J Am Chem Soc. 2008 August 13; 130(32): 10643–10647
15. P.S.sona, nanoparticulate drug delivery systems for the treatment of diabetes, Digest Journal of Nanomaterials and Biostructures, Vol. 5, No 2, April-June 2010, p. 411 – 418
16. Di Gianvincenzo P, Marradi M, Martínez-Avila OM, Bedoya LM, Alcamí J, Penadés S, Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents, Bioorg Med Chem Let. 2010 May 1;20(9):2718-21.
17.Alexander CM, Maye MM, Dabrowiak JC.DNA-capped nanoparticles designed for doxorubicin drug delivery. Chem Commun (Camb). 2011 Mar 28;47(12):3418-20
18. Hosta-Rigau L, Olmedo l, Arbiol J, Cruz LJ, Kogan MJ, Albiricio F, Multifunctionalized gold nanoparticles with peptides targeted to gastrin-releasing peptide receptor of a tumor cell line, Bioconjug. Chem. 2010 Jun 16;21(6):1070-8.
19. Qin G, Li Z, Xia R, Li F, O'Neill BE, Goodwin JT, Khant HA, Chiu W, Li KC Partially polymerized liposomes: stable against leakage yet capable of instantaneous release for remote controlled drug delivery Nanotechnology, 2011 Apr 15;22(15):155605.
20. Wang F, Wang YC, Dou S, Xiong MH, Sun TM, Wang J, Doxorubicin-Tethered Responsive Gold Nanoparticles Facilitate Intracellular Drug Delivery for Overcoming Multidrug Resistance in Cancer Cells, ACS Nano. 2011 Apr 4.
21. Chen Y.-H. Tsai C.-Y, Huang P.-Y.,. Chang M.-Y, Cheng P.-C., Chou C.-H, Chen D.-H.,. Wang C.-R. Shiau, A.-L, Wu C.-L., Methotrexate conjugated to gold nanoparticles Inhibits tumor growth in a syngeneic lung tumor model, Mol. Pharm. 4 (5) (2007)713–722
22. Huschka R, Neumann O, Barhoumi A, Halas NJ, Visualizing light-triggered release of molecules inside living cells, Nano Lett. 2010 Oct 13; 10(10):4117-22.
23. Huang Y, Yu F, Park YS, wang J, Shin MC, Chung HS, Yang VC, Co-administration of protein drugs with gold nanoparticles to enable percutaneous delivery, Biomaterials, 2010 Dec;31(34):9086-91
24. Niidome T, Shiotani A, Akiyama Y, Ohga A, Nose K, Pissuwan D, Niidome Y, Theragnostic approaches using gold nanorods and near infrared light, Yakugaku Zasshi, 2010 Dec;130(12):1671-7
25. Kim D, Jeong YY, Jon S, A drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer, ACS Nano, 2010 Jul 27; 4(7):3689-96
26. Jain K.K., Advances in the field of nanooncology, BMC Med. 2010; 8: 83
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org









