 About Author:
About Author:
K.Kranthi Kumar
SKU College of Pharmaceutical Sciences,
S.k.univrsity, Anantapur
kranthikumarkotta@gmail.com
Abstract
Molecular farming officially known as transgenic non-food GM plant pharming and biopharming, is a type of genetic modification used in farming involving the use of plants, and potentially also animals, as the means to produce compounds of therapeutic value. The idea is to use such crops as biological factories to generate drugs difficult or expensive to produce in any other way. The issue of genetically modified crops has been around for a number of years and continues to be a controversial subject.
REFERENCE ID: PHARMATUTOR-ART-1928
History
Edible vaccines were first tested on humans in 1997, when scientists asked volunteers to eat anti-diarrhoea potatoes produced by the Boyce Thompson Institute at Cornell University, Ithaca, NY, USA. Roswell Park Cancer Institute in Buffalo, New York also developed edible vaccines in raw potatoes and foreign proteins (HBs IgA) can help to cure human being from Hepatitis B virus Molecular biologist of the London Health Sciences Centre developed edible vaccines to combat autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, lupus and even rejection The first recombinant plant-derived pharmaceutical protein (PDP) was human serum albumin, initially produced in 1990 in transgenic tobacco and potato plants. Fifteen years on, the first technical proteins produced in transgenic plants are on the market, and proof of concept has been established for the production of many therapeutic proteins, including antibodies, blood products, cytokines, growth factors, hormones, recombinant enzymes and human and veterinary vaccines. Furthermore, several PDP products for the treatment of human diseases are approaching commercialization, including recombinant gastric lipase for the treatment of cystic fibrosis, and antibodies for the prevention of dental caries and the treatment of non-Hodgkin's lymphoma. There are also several veterinary vaccines in the pipeline; Dow Agro Sciences announced recently their intention to produce plant-based vaccines for the animal health industry.
Introduction
Vaccines are primary tools in programmes for health intervention for both humans and animals. They would be more widely used especially in developing countries. It would be helpful for human society if cost of production could be reduced and they could be distributed without refrigeration. Vaccines couldn’t be very popular because of unavailability of electricity for its storage in remotest area in developing countries.
Vaccines and antibodies play a key role in healthcare. However, the cost of production and maintaining a chain for vaccine distribution has so far hampered realizing their full potential. Expression of antigens as vaccines and of antibodies against antigens of pathogens in transgenic plants is a convenient and inexpensive source for these immunotherapeutic molecules.
Research underway is dedicated to solving these limitations by finding way to produce oral (edible) vaccines in transgenic plants. Edible vaccines can be produced by transgenic plants in large amount and cost will be also cheap and no problem of refrigeration and all section of people can afford to buy it for remedy of a large number of diseases Hepatitis B virus (HBV) infection is probably the single most important cause of persistent viremia in humans.
The disease is characterized by acute and chronic hepatitis, which can also initiate hepatocellular carcinoma. The prevalence of the disease in developing countries justified initial efforts to express HBV candidate vaccines in plants. Currently two forms of HBV vaccines are available, both of which are injectable and expensive- one purified from the serum of infected individuals and the other a recombinant antigen expressed and purified from yeast.
This antigen has already been entered in transgenic plant either through Agrobacterium mediated transformation or Particle gun bombardment (Biolistics) and encoding the hepatitis B surface antigen (HBsAg); this is the same antigen used in the commercial yeast-derived vaccine. An antigenic spherical particle was recovered from these plants which is analogous to the recombinant hepatitis surface antigen (HBsAg) derived from yeast. Parenteral immunization of mice with the plant–derived material has demonstrated that it retains both B and T-cell epitopes, as compared to the commercial vaccine.
Diarrhoeal disease causes up to 10 million deaths per year in the developing world, primarily among children. Relatively little research to prevent these diseases is underway, as they represent more of a nuisance than a severe problem in developed countries. Studies supported by the World Health Organization have demonstrated an effective vaccine for cholera, which provides cross protection against enterotoxic Escherichia coli. This vaccine is not available, however, in large part due to cost of production of the bacterial toxin protein which is a component of its formulation.
To address this limitation, plants were transformed with the gene encoding the B subunit of the E.coli heat labile enterotoxin (LT-B). Transgenic potatoes expressing LT-B were found to induce both serum and secretory antibodies when fed to mice; these protective in bacterial toxin assays in vitro. This is the first “proof of concept” for the edible vaccine.
The selection of a plant system for delivery of edible vaccines for humans has been addressed. Recognizing that it is necessary to express the desired protein in a food that is consumed raw (to avoid denaturation of the candidate vaccine protein), a system to transform banana plants has been developed. The expression of candidate vaccines in banana fruit will be dependent upon identification of suitable specific promoter to drive the desired gene expression. Research to find these genetic regulatory elements are now underway.
Edible vaccine research is currently directed at human diseases, with a special emphasis on the developing world. The technology will also have immediate value for the production of inexpensive vaccines as food additives for agricultural animals. Since various plant tissues are fed to animals, other plants such as alfalfa, maize and wheat could be valuable vehicles to deliver vaccines (and perhaps pharmaceuticals) for the betterment of animal health.
Recent progress in the area of transgenic plants has however, once again attracted attention of the scientists, and plants are being looked upon as potential bio-reactors or bio-factories for the production of immunotherapeutic molecules.
In 1989 firstly Hiatt and co-workers attempted to produce antibodies in plants which could serve the purpose of passive immunisation but it was appeared in 1990 in the form of patent application, the concept of edible vaccine got impetus after Arntzen and co-workers expressed hepatitis B surface antigen in tobacco in 1992 to produce immunologically active ingredient via genetic engineering of plants. This generated a good deal of excitement among biotechnologists, particularly in light of the potential of edible vaccines and antibodies for immunotherapy for countries like India.
Various strategies for expression of foreign genes in high amounts in plants include use of strong and organ specific plant promoters, targeting of the protein into endoplasmic reticulum (ER) by incorporating ER-targeting and ER-retention signals, creation of optimized translation start site context as well as alteration of codons to suit the expression of prokaryotic genes in a plant. Though promoters of genes, like maize ubiquitin and rice actin, have been reported to direct high level of expression in monocots, the 35S promoter of cauliflower mosaic virus remains the promoter of choice for dicots . Targeting of the protein to appropriate cellular compartment may be helpful in stabilizing the protein. Retention of heat labile E.coli enterotoxin in ER of potato by using ER-retention signal has been reported to elevate the expression levels of the recombinant protein.
Though signals for membrane targeting, protein folding, oligomerization and N-glycosylation are highly conserved in animals and plants, while expressing bacterial proteins targeted to ER, it is important to consider the sequence of a signal peptide for targeting to periplasmic space in bacterium may not be equally efficient in plants. Substitution of signal peptide of bacterial origin with a plant specific ER-targeting sequence was observed to dramatically increase the glycosylation and secretion efficiency of chitinase.
For production of edible vaccines or antibodies, it is desirable to select a plant whose products are consumed raw to avoid degradation during cooking. Thus, plants like tomato, banana and cucumbers are generally the plants of choice. While expression of a gene that is stably integrated into the genome allows maintenance of the material in the form of seeds, some virus based vectors can also be used to express the gene transiently to develop the products in a short period. This may have the additional advantage of allowing expression of the product at very high level; not always attainable in transgenic systems.
While plant system may have the capability of producing any vaccine in large amounts and in a less expensive manner, purification of the product may require the use of existing or even more cumbersome procedures. Attention therefore has been paid to mainly those antigens that stimulate mucosal immune system to produce secretory IgA (S-IgA) at mucosal surfaces, such as gut and respiratory epithelia.
In general, a mucosal response is achieved more effectively by oral instead of parenteral delivery of the antigen. Thus, an antigen produced in the edible part of a plant can serve as a vaccine against several infectious agents which invade epithelial membranes. These include bacteria and viruses transmitted via contaminated food or water, and resulting in diseases like diarrhoea and whooping cough.
The first report of the production of edible vaccine (a surface protein Streptococcus) in tobacco, at 0.02% of total leaf protein level appeared in 1990 in the form of a patent application published under the International Patent Cooperation Treaty. Subsequently, a number of attempts were made to express various antigens in plants. Hein is one of handful researchers using the tools of bioengineering to transform ordinary fruits and vegetables into botanical cargo vessels that carry life saving vaccines. Edible vaccines promise to be an affordable and safe way for people in even the most poverty stricken parts of the world to protect themselves against disease. Normal vaccines need for refrigeration and purified serum, hypodermic needles, or even trained medical professional to distribute and oversee vaccinations but these conditions are not required for edible vaccines.
The goal is to give people in developing countries the genetically engineered seeds that will sprout edible vaccines. “Every culture on this planet raises food” explains Hein. “This can provide developing countries with a stable vaccine source because it will be genetically coded into the food”. Using recombinant DNA technology, researchers can now isolate the genes—called antigens—that mobilize our natural defences.
But impregnating plants with these antigens requires an impressive bit of molecular legerdemain. At Scrips Research Institute, for instance, the antigen is snipped off the deadly cholera pathogen. Then it is inserted into the cells of a bacterium that causes a plant disease called crown gall. The alfalfa plants are infected with these transgenic crown gall organisms, which can penetrate the plant’s cell walls. The plant cells containing the foreign genes are then cultured in a Petri dish until they are mature enough to be transplanted.
The next step is to test the potency of the antigens in plants raised in the field, outside of the cloistered laboratory. “We’ve just harvested this crop of alfalfa” says Hein, who’s in the midst of measuring its antigen level and feed this transgenic grain to mice.
Antigens produced in transgenic plants
|
Protein |
Plant |
|
Hepatitis B surface antigen Rabies virus glycoprotein Norwalk virus capsid protein E.coli heat-labile enterotoxin B subunit Cholera toxin B subunit Mouse glutamate decarboxylase VP1 protein of foot and mouth disease virus Insulin Glycoprotein swine-transmissible gastroenteritis cornavirus
|
Tobacco Tomato Tobacco Potato Potato, tobacco Potato Arabidopsis Potato Arabidopsis |
Since acute watery diarrhoea is caused by enteroxigenic Escherichia coli and Vibrio cholerae that colonize the small intestine and produce one or more enterotoxin (LT-B) in tobacco and potato . The enterotoxin (LT) from E.coli is a multimeric protein, quite similar to cholera toxin (CT) structurally, functionally and antigenically. LT has one A subunit (27 kDa) and a pentamer of B subunits (11.6 kDa). Binding of the non-toxic LT-B pentamer to GM1 gangliosides, present on epithelial cell surfaces, allows entry of the toxin LT-A subunits into the cells. LT-B and CT-B are both potent oral immunogens. An oral vaccine composed of the cholera toxin–B subunit (CT-B) with killed Vibrio cholera cells has been reported to give significant level of protection against cholera . But the cost of production of CT-B by conventional methods is too high to allow distribution of this vaccine.
The recombinant LT-B (rLT-B) produced in tobacco and potato showed partial pentamerization after the engineering of subunit gene in a way that allowed retention of the protein in microsomal vesicles. On testing immunogenicity of rLT-B by feeding potato tubers to mice, both humoral and mucosal immune responses were reported to be stimulated. This vaccine has gone through pre-clinical trials in humans. The antigenic protein retained its immunogenicity after purification from the transgenic potato expressing it . Fourteen healthy individuals, who ate 50-100 g raw potatoes, were screened for gut-derived antibody secreting cells, which were detectable 7-10 days after immunization. Presence of both anti-LT IgA-secreting cells and anti-LT IgG-secreting cells was detected in the peripheral blood.
Cholera toxin, which is very similar to E.coli LT, has also been expressed in plants, generated tobacco plants expressing CT-A or CT-B subunits of the toxin. CT-A produced in plant was not cleaved into A1 and A2 subunits, which happens in epithelial cells. Plants expressing CT-B showed the presence of a protein that migrated to the same position in denaturing gel as the CT-B derived from V.cholerae, and was recognised by mouse anti-CT-B antibody. Cholera toxin-B subunit, when expressed in potato, was processed in a natural way; the pentameric form (the naturally occurring form) being the abundant form. Antigenically it was found to be similar to the bacterial protein. Even after boiling transgenic potato tubers till they became soft, approximately 50% of the CT-B was present in the pentameric GM1 ganglioside binding form .
Similarly, a rabies virus coat glycoprotein gene has been expressed in tomato plants . The protein that was expressed had molecular mass of 62 kDa. Since the orally administered protein elicited protected immunity in animals, it was expected that continued efforts would lead to development of an edible oral vaccine against rabies which could be used as a preventive strategy.
The Hepatitis B surface antigen (HBsAG) has been reported to accumulate to 0.01% of soluble protein level in transgenic tobacco . The antigens, delivered in a macromolecular form, are known to survive the gut atmosphere and perform better. Carrillo et al 1998 expressed structural protein, VP1, of foot and mouth disease virus in Arabidopsis. The mouse that was immunized intra-peritoneally with a leaf extract elicited immune response to synthetic peptides carrying various epitopes of VP1, or to complete VP1. Furthermore, all the mice immunized with the leaf extract were protected against challenge with virulent foot and mouth disease virus.
One of the alternative strategies of producing a plant-based vaccine is to infect the plants with recombinant viruses carrying the desired antigen that is to infect the plants with recombinant viruses carrying the desired antigen that is fused to viral coat protein. The infected plants have been reported to produce the desired fusion protein in large amounts in a short time.
The technique involved either placing the gene downstream a subgenomic promoter or fusing the gene with capsid protein that coats the virus (Table-2). The latter strategy is perhaps the strategy of choice since fusion proteins in particulate form are highly immunogenic. Edible vaccines offer exciting possibilities for significantly reducing the burden of diseases like hepatitis and diarrhoea, particularly in the developing world where storing and administering vaccines are often major problems. Baltimore, Maryland, April 28, 1998 opening a new era in vaccine delivery, researchers supported by the National Institute of Allergy and Infectious diseases (NIAID) have shown for the first time that an edible vaccine can safely trigger significant immune responses in people.
Transient production of antigens in plants after infection with plant viruses expressing a recombinant gene
|
Protein |
Plant |
carrier |
|
Influenza antigen |
Tobacco |
TMV |
|
Murine zona pellucida antigen |
Tobacco |
TMV |
|
Rabies antigen |
Spinach |
AFMV |
|
HIV-1 antigen |
Tobacco |
AFMV |
|
Mink enteritis virus antigen |
Black eyed bean |
CPMV |
|
Colon cancer antigen |
Tobacco |
TMV |
AFMV, alfalfa mosaic virus; TMV, tobacco mosaic virus; CPMV, cowpea mosaic virus.
After consuming the potatoes volunteers produced antigens in their bodies just as if they had received a traditional anti-diarrhoeal vaccine and they experienced no adverse side effects.
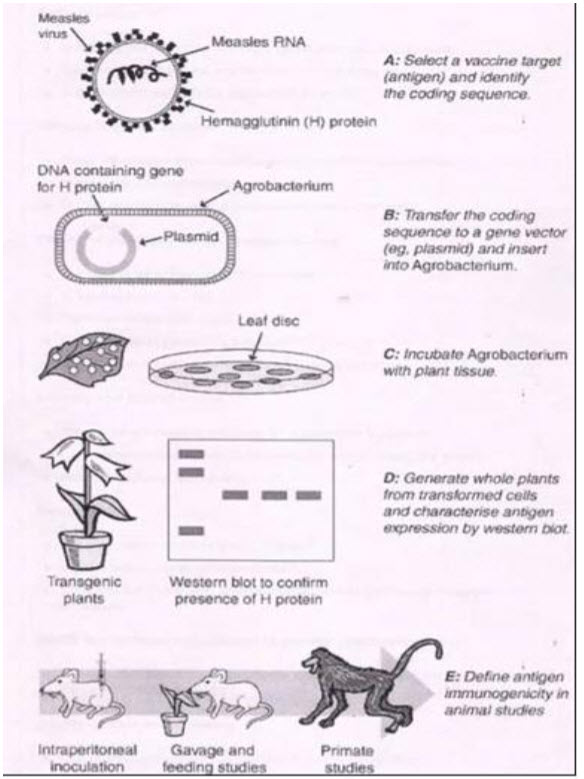
Globally, measles causes over 800,000 deaths every year (Centres for Disease Control and Prevention, 1998). Many other affected people either become deaf or are weakened by pneumonia or encephalitis. The vaccine currently available for measles has been used effectively and safely since the 1960s and results in 95% seroconversion in individuals who are over the age of 18 months at the time of vaccination (15). However, the measles live-attenuated vaccine (LAV) has no oral efficacy and is destroyed by heat, so that its distribution and storage are dependent on maintenance of a “cold-chain” of refrigeration. Finally, the effectiveness of the LAV is reduced by the presence of maternal antibodies (51). These limitations present a serious challenge to the goal of measles eradication.
Development of an edible measles vaccine- The first stage in the development of an edible vaccine is selecting which antigen to express. Measles is an enveloped virus with two major surface proteins- the hemagglutinin (H) and fusion proteins. Antibodies raised to the H protein after infection with the wild type measles virus (MV) have MV-neutralising activity and correlate with immunological protection (12). The H protein subunit from the attenuated Edmonston vaccine strain was therefore selected as the basis for an edible measles vaccine Edible vaccines are currently being developed for a number of human and animal diseases, including measles, cholera, foot and mouth disease, and hepatitis B & C. Many of these diseases are likely to require booster vaccinations or multiple antigens to induce and maintain protective immunity. Plants have the capacity to express more than one transgene, allowing delivery of multiple antigens for repeated inoculations.
Preparation methods:
Transgenic plants may be produced by a number of methods. The most common uses Agrobacterium tumefaciens, a naturally occurring soil bacterium, to transfer a small segment of DNA into the plant genome in a process known as transformation (Fig 1). Whole plants can then be regenerated from individual plant cells that have been successfully transformed.
Production of transgenic plants is species-dependent and can take three to nine months. By this method MV-H gene been successfully expressed in the experimental model plant tobacco. When given orally to mice, the transgenic plant extract containing the MV-H antigen induced serum antibodies that were able to neutralise wild type MV in vitro, showing that plant-derived MV-H protein retains its immunogenic.
Secretary IgA is indicative of a mucosal immune response, which is important for protection against diseases that establish infection through mucosal surfaces such as the respiratory tract. Plant can produce a large amount of recombinant proteins for remedy of different disease and act as factories .
Needle free vaccination programme could be initiated after the development of rice based mucosal vaccine .Vaccines have been developed in fruit which can be used during dinner and allergic and other immunotherapy could also be possible .
From model system to practical vaccine- The next challenge will be to translate this technology from a model system into a practical vaccine. While tobacco is a good model system for evaluating the production recombinant proteins, it produces toxic compounds which make it unsuitable for vaccine delivery. Clinical trials have shown the induction of immune responses with antigen expressed in potato and lettuce . Lettuce is a fast growing species suitable for direct consumption and experimental studies. Another practical alternative may be rice, which is commonly used in baby food because of its low allergenicity. Recent studies have shown that mammalian proteins can be expressed to high levels in transgenic rice . Furthermore, rice is easy to store and transport, and protein expressed in rice grains is stable at room temperature. Rice flour can also be mixed with baby food, clean water or breast milk for delivery to infants.
Strategy for the development of plant edible vaccine.
Modulation of immune response to acquire immune tolerance
One of the utilities of producing antigens in plants in large amount is in treatment of autoimmune diseases like diabetes mellitus which involve production of antibodies against glutamic acid decarboxylase (GAD) and insulin, leading to destruction of insulin-producing pancreatic cells . The antigens targeted for autoimmune response can be fed to the animals to induce immune tolerance. However, since the use of antigens for inducing oral tolerance requires production in large amounts of the human antigens that are generally difficult to produce by conventional means, attempts have been made to produce such antigens in plants. Insulin and GAD have been produced in potato and tobacco, respectively. To detect the delivery of plant-synthesized insulin to the gut associated lymphoid tissue, insulin was linked to cholera- toxin B subunit. Non-obese diabetic mice which were fed with the transformed potato tuber tissue containing microgram level of the recombinant insulin delayed the progression of clinical diabetes. Similarly, GAD-producing tobacco plants, given as a dietary supplement, inhibited the development of diabetes in the non-obese diabetic mouse.
Expression and assembly of antibodies in plants
Transgenic plants are also being looked upon as a source for producing large-scale antibodies which can serve the purpose of passive immunization by direct application, in addition to providing a tool for drug targeting or interactive inactivation of undesirable molecules . Gene technology has provided great impetus to the utility of antibodies, since antibody genes can be altered to order. Thus not only genes coding for both the light and heavy chains have been expressed, but modified genes capable of expressing only Fab fragments (assembled light chains and shortened heavy chains) or ScFV (single peptide chains where variable domains of heavy and light chains are covalently linked by a short flexible peptide) have also been expressed in bacteria and mammalian cells (Table-3). Murine antibodies have humanized by changing the constant and framework domains. In addition, recent technology involving PCR (Polymerase chain reaction) and phage display allow cloning and screening of antibodies with suitable avidity easily.
Antibodies and antibody fragments produced in transgenic plants
|
Antibody |
Antigen |
Plant |
|
IgG (k) |
Transition stage analog |
Tobacco |
|
IgM (λ) |
NP(4-hydroxy-3-nitrophenyl) acetyl hapten |
Tobacco |
|
Single domain (dAb) |
Substance P |
Tobacco |
|
Single chain Fv |
Phytochrome |
Tobacco |
|
Single chain Fv |
Artichoke mottled virus coat protein |
Tobacco |
|
Fab; IgG (k) |
Human creatin kinase |
Arabidopsis |
|
IgG (k) |
Fungal cutinase |
Tobacco |
|
IgG (k) and SIgG/A hydrid |
S. mutagens adhesin |
Tobacco |
|
Single chain Fv |
Abscisic acid |
Tobacco |
|
Single chain Fv |
Nematode antigen |
Tobacco |
|
Single chain Fv |
β-glucuronidase, β-1,4endoglucanase, Herpes simplex virus |
Tobacco |
Transgenic plants not only provide the means to express antibodies but also enable the glycosylation and entry into secretory pathway which allow assembly of complete antibodies and Fab fragments. Variable fragments (Fv) can be produced in cytosol, directed to different compartments and fused with proteins such as protein A and phosphatase to improve the detection and purification of single chain Fv (scFv). In plants, antibody production (1-5% of total plant protein) has been achieved by cross-pollination of individually transformed plants expressing light or heavy chains . Other approaches involve double transformation or transformation by constructs having genes for both light and heavy chains on the same vector. Despite the fact that production of antibodies in plants takes longer, the low cost of production and capability of increasing production simply by increased propagation make plant antibodies an attractive proposition.
Aiming at therapeutic treatment , have succeeded in producing multimeric secretory IgA (SIgA) molecules in plants which represent the predominant form of immunoglobulin in mucosal secretions. SIgA not only contains heavy and light chains but it is also dimerized by a J chain, and protected from proteolysis by a fourth polypeptide, the SC. Production of such antibodies in mammalian cells is very complex because of the requirement of B cell as well as gut epithelial cells for the formation of the SIgA. Thus, four transgenic tobacco plants were produced by genetic engineering which produced a murine monoclonal antibody light k chain, the hybrid IgA-G antibody heavy chain, murine J chain and rabbit secretory component.
A series of sexual crosses was carried out to allow expression of all the four proteins simultaneously. The progeny produced a functional secretory immunoglobulin very efficiently. This demonstrated the potential of plants in assembly of antibodies, and the flexibility of system. Recently, a humanized monoclonal antibody against glycoprotein B of herpes simplex virus 2 (HSV-2) has been expressed in soybean. This antibody was found to possess the same efficacy for prevention of vaginal HSV-2 infection in mice and similar stability in human semen as the antibody expressed in human cell culture and also plant cell cultures .
Topical application of antibodies has already been shown to control infection by way of passive immunization. A hybrid monoclonal antibody (IgA/G), having constant regions of IgG and IgA fused, has been used successfully against human dental caries caused by the bacterium Streptococcus mutans . Ma et al. (1998) compared the secretory antibody produced in transgenic tobacco (SIgA/G) and the original mouse IgG. Though both had similar binding affinity to surface adhesion protein of S.mutans, SIgA/G survived for three days in the oral cavity, whereas IgG could survive for just one day. The plant antibody provided protection against the colonization of the S. mutans for at least four months. These results show that this strategy could be useful for many other mucosal infections in humans and animals. Medical molecular farming has been done for the production of antibodies, biopharmaceuticals and edible vaccines in plants for immunotherapy. Co-expression of soybean glycinias A1 aB 1b and A3B4 enhances their accumulation levels in transgenic rice seeds.
Evaluation:-
In vivo studies:-
The goal of the study was to demonstrate that an edible vaccine could stimulate an immune response in humans. Volunteers ate bite sized pieces of raw potato that had been genetically engineered to produce part of the toxin secreted by the Escherichia coli bacterium, which causes diarrhoea. It also showed that transgenic potatoes containing this segment of the toxin stimulated strong immune responses in animals. The trial enrolled 14 healthy adults; 11 were chosen at random to receive the genetically engineered potatoes and three received pieces of ordinary potatoes. The investigators periodically collected blood and stool samples from the volunteers to evaluate the vaccine stimulate both systemic and intestinal immune responses. Ten of the 11 volunteers (91%) who ingested transgenic potatoes had fourfold rises in serum antibodies at some point after immunization, and six of five (the 91%) developed fourfold rises in intestinal antibodies. The potatoes were well tolerated and no one example for serious adverse side effects. Encouraged by the results of this study, NIAID- supported scientists are exploring the use of this technique administering other antigens. Edible vaccines for other intestinal pathogens are already in the pipeline- for potatoes and bananas that might protect against Norwalk virus, a common cause of diarrhoea, and potatoes and tomatoes that might protect against Hepatitis B. This first trial is a map road to creating inexpensive vaccines that might be particularly useful in immunizing people in developing countries where high cost and logistic issues, such as transportation and the need for certain vaccines to be refrigerated thwart effective vaccination programmes. The study nurse at the University of Maryland peeled the potatoes just before they were eaten, because potatoes sometimes contains a compound that imparts a bitter taste and can cause nausea and stomach upset. The potatoes then cut into small, uniform pieces and weighed into 50-gram and 100-gram doses. Each person received either 50grams or 100 grams of potato over a three-week period, 0, 7 and 21 days. The dosage size varies evaluate any side effects from eating raw potatoes.
NIAID is a component of the National Institutes of Health (NIH). NIAID conducts and supports research to prevent, diagnose and treat illnesses such as AIDS and other serious transmitted diseases, malaria, tuberculosis, asthma and allergies; NIH is an agency of the U.S. Department and Human Services. At least 350 genetically engineered pharmaceutical products are currently developed in the United States and Canada. This is a welcome step towards the new world of molecular farming. Plant based biopharmaceuticals have also been produced and expressed . Edible vaccine could show multiple T cell epitopes for oral tolerance against antigens
Advantages:
Plants do not carry pathogens that might be dangerous to human health. Additionally, on the level of pharmacologically active proteins, there are no proteins in plants that are similar to human proteins. On the other hand, plants are still sufficiently closely related to animals and humans that they are able to correctly process and configure both animal and human proteins. Their seeds and fruits also provide sterile packaging containers for the valuable therapeutics and guarantee a certain storage life.
Global demand for pharmaceuticals is at unprecedented levels, and current production capacity will soon be overwhelmed. Expanding the existing microbial systems, although feasible for some therapeutic products, is not a satisfactory option on several grounds. First, it would be very expensive for the pharmaceutical companies. Second, other proteins of interest are too complex to be made by microbial systems. These proteins are currently being produced in animal cell cultures, but the resulting product is often prohibitively expensive for many patients. Finally, although it is theoretically possible to synthesize protein molecules by machine, this works only for very small molecules, less than 30 amino acid residue in length. Virtually all proteins of therapeutic value are larger than this and require live cells to produce them. For these reasons, science has been exploring other options for producing proteins of therapeutic value
Disadvantages:
While molecular farming is one application of genetic engineering, there are concerns that are unique to it. In the case of genetically modified (GM) foods, concerns focus on the safety of the food for human consumption. In response, it has been argued that the genes that enhance a crop in some way, such as drought resistance or pesticide resistance, are not believed to affect the food itself. Other GM foods in development, such as fruits designed to ripen faster or grow larger, are believed not to affect humans any differently from non-GM varieties.
In contrast, molecular farming is not intended for crops destined for the food chain. It produces plants that contain physiologically active compounds that accumulate in the plant’s tissues. Considerable attention is focussed, therefore, on the restraint and caution necessary to protect both consumer health and environmental biodiversity.
There are also problems associated with the use of plants as protein bioreactors. Plant proteins have different sugar residues from human or animal proteins. Freiburg-based greenovation Biotech GmbH, in cooperation with Professor Ralf Reski’s research group at the University of Freiburg, has shown that this problem can be solved through the use of Physcomitrella patens. Because the scientists cultivate the moss in tube-shaped photobioreactors in a liquid medium, they have no worries that the genetically modified mosses might be released into the environmentScope for future study
Scope for future study:
Vaccines have been one of the most far-reaching and important public health initiatives of the 20th century. Advancing technology, such as oral DNA vaccines, intranasal delivery and edible plant derived vaccines, may lead to a future of safer and more effective immunization.
Edible vaccines, in particular, might overcome some of the difficulties of production, distribution and delivery associated with traditional vaccines. Significant challenges are still to be overcome before vaccine crops can become a reality. However, while access to essential healthcare remains limited in much of the world and the scientific community is struggling with complex diseases such as HIV and malaria, plant derived vaccines represent an appetising prospect.
The potential advantages of plant based vaccines are edible means of administration, reduced need for medical personnel and sterile injection conditions, economical to mass produce and transport, reduced dependence on foreign supply, storage near the site of use, heat stable, eliminating the need for refrigeration, antigen protection through bioencapsulation, subunit vaccine (not attenuated pathogens) means improved safety, seroconversion in the presence of maternal antibodies, generation of systemic and mucosal immunity, enhanced compliance (especially in children), delivery of multiple antigens, and integration with other vaccine approaches.
References:
1. Arakawa, T., Chong, D.K.X., and Langridge, W.H.R. (1997). Expression of Choleratoxin B subunit oligomers in transgenic potato plants, Transgenic Res, 6: 403-413
2. Arakawa, T., Chong, D.K.X. and Langridge, W.H.R. (1998). Transgenic plants for the production of edible vaccine and antibodies for immunotherapy, Nature Biotechnol, 16: 292-297
3. Arakawa, T., Yu, J. and Chong, D.K.X. (1998). A plant based cholera toxin B subunit insulin fusion protein protects against the development of autoimmune diabetes, Nat. Biotechnol., 16: 934-938
4. Arntzen, C.J. (1998). Pharmaceutical foodstuffs-oral immunization with transgenic plants, Nature Medicine (supplement), 4: 502-03
5. Artsaenko, O., Peisker, M., Zurniedan, U., Fielder, U., Weiler, E.W., Muntz, K., Conrad, U. (1995). Expression of a single chain FV antibody against abscisic acid creats a wilty phenotypes in transgenic tobacco, Plant J., 8: 745-750
6. Baum, T.J., Hiatt, A., Parrott, W.A., Pratt, L.H., Hussey, R. S. (1996). Expression in tobacco of a monoclonal antibody specific to secretion of the root knot nematode, Mol. Plant Microbe Interact., 9: 382-387
7. Benvenuto, E., Ordas, R.J., Tavazza, R., Ancora, G., Biocca, S., Cattaneo, A. and Galeffi, P. (1991). Phytoantibodies: A general vector for the expression of immunoglobular domains in transgenic plants, Plant Mol. Biol., 17: 865-874
8. Carter, J.E.III and Langridge, W.H.R. (2004). Plant based vaccine for protection against infectious and autoimmune diseases, Crit. Rev. Plant Sci., 21: 93-109
9. Carrillo, C., Wigdorovitz, A., Oliveros, J.C., Zamorano, P.I., Sadir, A.M., Gomez, N., Salinas, J., Escribano, J.M. and Borca, M.V. (1998). Expression of immunogenic S1 Glycoprotein of infectious Bronchitis virus in transgenic potatoes, J.Virol., 72: 1688-1690
10. Carrillo, C.A., Wigdorovitz, K., Trono, M.J., Dus Santos, S., Castanon, A.M., Sadir, R., Ordas, J.M., Escribano, J.M., Borca, M.V. (2001). Induction of a virus-specific antibody response to foot and mouth disease virus using the strain VP1 expressed in transgenic potato plants, Viral Immunol., 14:49-57
11. Centers for Disease Control and Prevention Global measles control and regional elimination, 1998-1999, MMWR Morb Mortal Wkly Rep. 1999, 48: 1124-1130
12. Chen, R. T., Markowitz, L.E., Albrecht, P. (1999). Measles antibody re-evaluation of protective titres , J. Infect Dis, 162 ; 1036-1042
13. Chrispeels, M.J. and Tague, B.W. (1991). Protein sorting in the secretory system of plant cells, Int. Rev. Cytol.,125: 1-45
14. Conrad, U.; Fiedler, U. (1994). Expression of engineered antibodies in plant cells, Plant Mol. Biol . , 26:1023-1030
15. Cutts, F., Henao-Restrepo, A.M., Olive, J. (1999). Measles elimination: progress and challenges, Vaccine, 17: S47-S52
16. Daniell, H., Streatfield, S.J. and Wycoff, K. (2001). Medical molecular farming production of antibodies, biopharmaceuticals and edible vaccines in plants, Trends Plant Sci., 6: 219-226
17. DeAizpura, H.J., Wilson, Y.M. and Harrison, L.C. (1992). Transgenic plants for the production of edible vaccine and antibodies for immunotherapy, Proc. Natl. Acad. Sci. USA, 89: 9841-9845
18. De Neve, M., De Loose, M., Jacobs, A., Van Houdt, H., Kaluza, B., Weidle, U. and Depicker, A. (1993). Silencing of antibody genes in plants with single copy transgene inserts as a result of gene dosage effects, Plant Mol.Biol ., 2: 227-237
19. During, K., Hippe, S., Kreuzaler, F. and Schell, J. (1990). Transgenic plants expressing a function single-class FV antibodyare specifically protected from virus attach, Transgenic Res., 15: 281-293
20. Fischer, R., Stoger, E., Schillberg, S., Christou, P. And Twyman, R.M. (2004). Plant-based production of biopharmaceuticals, Curr. Opin. Plant Biol., 7: 152-158
21. Gomez, N., Carrillo, G.N., Salinas, J., Parra, F., Borca, M.V.and Escribano, J.M.(1998). Expression of immunogenic glycoproteins 3 polypeptides from trans Virology, 249: 352-358
22. Haq, T.A., Mason, H.S., Clements, J.D.and Arntzen, C.J.(1995). Oral immunization with a recombinant bacterial antigen produced in transgenic plants, Science, 268:714-716
23. Hellwig, S., Drossard, J., Twyman, R.M. and Fischer, R. (2004). Plant cell cultures for the production of recombinant proteins, Nature Biotechnol,22: 1415-14
24. Hein, M.B., Yeo, T.C., Wang, F. and Sturtevant, A. (1996). Expression of cholera toxin subunits in plants, Ann. NY Acad. Sci., 792 : 51-56
25. Hidenori, T., Takachika H., Lijun, Y., Yoshifumi, T., Yoshikazu, Y., Karoru, T., Ryotaro, I., Hideyuki, K., Hiroshi, K. And Fumio, T. (2005). A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2- mediated IgE responses, Proc. Natl. Acad. Sci. U.S.A., 102: 17255-17530
26. Hiatt, A., Cafferkey, R and Bowdish, K (1989). Production of antibodies in transgenic plants, Nature, 342: 76-78
27. Hiatt, A. and Mostov, K. (1993). In Transgenic Plants: Fundamentals and Applications (ed. Hiatt. A), Marcel Dekker, Inc, New York, pp. 221-237
28. Hidenoi, T. (2009). Oral edible vaccine for allergy vaccines, National Kidney Foundation 2009 Spring Symposium, UK
29. Huang, Z., Dry, I., Webster, D (2001). Plant derived measles virus hemagglutinin protein induces neutralizing antibodies in rice, Vaccine, 19: 2163-217
30. Kapusta, J., Modelska, A., Figlerowicz, M (1999). A plant derived edible vaccine against hepatitis B virus, FASEB J., 13: 1796-1799
31. Kawakatsu, T., Yamamoto, M.P., Hirose, S., Yano, M., Takaiwa, F. (2008). Characterization of a new rice gluteilin gene GluD-1 expressed in the starchy endosperm, J.Exp. Bot., 1: 261-265
32. Kong, Q., Richter, L., Yang, Y.F., Arntzen, C.J., Mason, H.S. and Thanawala, Y. (2001). Oral immunization with hepatitis B surface antigen expressed in transgenic plants, Proc. Natl. Acad. Sci., USA, 98: 11539-11544
33. Longstaff, M., Newell, C.A., Boonstra, B., Strachan, G., Learmonth, D., Harris, W.J., Porter, A.J. and Hamilton, W.D. (1998). Expression and characterization of single chain antibody fragments produced in transgenic plants against the organic herbicides atrazine and panaquate, Biochem. Biophys. Acta, 1381: 147-160
34. Lund, P. and Dunsmuir, P. (1992). A plant signal sequence enhances the secretion of bacterial ChiA in transgenic tobacco, Plant Mol. Biol., 18: 47-53
35. Ma, S.W., Zhao, D.L., Yin, Z.Q., Mukherjee, R., Singh, B, Quin, H.Y., Stiller, C.R. and Jevnikar, A.M. (1997). Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance, Nature Med., 3: 793-796
36. Ma, J.K-C., Drake, P.M.W., Christou, P.(2003). The production of recombinant pharmaceutical proteins in plants, Nature Reviews/Genetics, 4: 794-805
37. Mason, H.S. and Arntzen, C.J. (1995). Transgene plants as vaccine production systems, Trends Biotechnol., 13: 388-392
38. Mason, H.S., Lam, D.M. and Arntzen, C.J. (1992). Expression of hepatitis B surface antigen in transgenic potato, Proc.Natl. Acad Sci. USA, 89: 11745-11749
39. Mason, H.S., Ball, J.M., Shi. J.J., Jiang, X., Estes, M.K. and Arntzen, C.J. (1996). Expression of Norwalk viral protein in transgenic potato and protein and its immunogenicity in mice, Proc. Natl. Acad. Sci. USA, 93: 5335-5340
40. Mason, H.S., Haq, T.A., Clements, J.D. and Arntzen, C.J. (1998). Edible vaccine protects mice against E.coli heat labile toxin (LT): potatoes expressing a synthetic LT-B gene, Vaccine , 16: 1336-1343
41. Ma, J.K. and Hein, M.B. (1995). Antibody production in plants, Trends Biotechnol., 13: 522-527
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









