About Authors:
Nimish Talaviya1*, Nirav Chandegara2, Rakesh Radadiya3, Chetana Ribadiya4, Chandani Joshi5, Dhara Kanjaria6
1,5Department of Q.A., Smt. R. D. Gardi B. Pharmacy College, Rajkot
2Department of Pharmacology, K. B. Institute of Technology, Gandhinagar
3Department of Q.A., Mts. V.B. Manvar College of Pharmacy, Dumiyani
4Department of Q.A., Smt. R. B. Patel Mahila Pharmacy College, Atkot
6Department of Q.A., Shree H. N. Shukla Institute of Pharmaceutical Education & Research, Rajkot
*nimish.talaviya@gmail.com
Abstract
This paper presents a RP-HPLC method for the simultaneous estimation of Paracetamol and Pamabrom in its synthetic mixture. The process was carried out on C18 (250 x 4.6mm, 5µ) (Spincotech Pvt. Ltd.) column using Phosphate buffer pH 4.5: Acetonitrile (75:25 %v/v) where pH was maintained using 0.1% orthophosphoric acid as a mobile phase at a flow rate of 0.8ml/min. Detection wavelength was fixed at isobestic point 268 nm. Linearity was observed in the concentration range of 10-50 μg/ml for Paracetamol (r2=0.9986) and 15-75 μg/ml for Pamabrom (r2=0.9971). The retention time of Paracetamol and Pamabrom was found to be 2.665 and 6.907 minutes respectively with resolution 13.70. The developed method is simple, precise, specific, robust, rapid, reproducible, and sensitive and it can be used for estimation ofParacetamol and Pamabrom in its synthetic mixture.
REFERENCE ID: PHARMATUTOR-ART-1943
INTRODUCTION
Paracetamol (PCM)is chemically 4-hydroxyacetanilide (Figure 1-A) used as analgesic and antipyretic. [1]Paracetamol acts primarily in the CNS, increasing the pain threshold by inhibiting both isoforms of cyclooxygenase, COX-1, COX-2 and COX-3 enzymes involved in prostaglandin (PG) synthesis. [2]
Pamabrom (PAM) is chemically 8-Bromo-3,7-dihydro-1,3-dimethyl-1-H-purine-2;6-dione compound with 2-amino-2-methyl-1-propanol (1:1) (Figure 1-B) is a diuretic drug. [3]Pamabrom is xanthine diuretics reduce salt and water reabsorption in the proximal tubule and increase renal blood flow, yet their diuretic effect is modest.[4]
The therapeutic importance of these two compounds justifies establishing analytical methods for its determination in bulk and laboratory mixture.
The chemical structures of Paracetamol and Pamabromare shown in Figure 1 (A), (B). [1, 3]
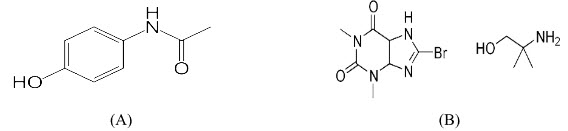
Figure-1: Chemical structure of (A) Paracetamol and (B) Pamabrom
Paracetamol is official in IP, BP and USP and is estimated by UV-Visible Spectrophotometric method as per IP, USP and BP. [3, 5, 6]In BP a redox titration for PCM is given for drug substance.[6]Pamabrom is official in US Pharmacopoeia. [3]It is assayed by Liquid chromatography as per USP. Literature review also reveals HPLC, UV spectrophotometric and HPTLC method for the estimation of PCM with other drugs. [7, 8, 9]Literature survey does not reveal any simple spectrophotometric method of Paracetamol and Pamabrom in synthetic mixture or Pharmaceutical dosage form. So the objective of this work was to develop simple, precise and rapid spectrophotometric methods for combined synthetic mixture containing Paracetamol, Pamabrom and excipients.
MATERIALS AND METHODS
Instrumentation
Isocratic HPLC system with LC-20AD pump, Hamilton injector with injection volume 20μL, Phenomenex C18(Luna 5micron, 250×4.6mm,i.d) column, Double beam spectrophotometer of Thermo Electron Corporation (HeλIOS α), Digital pH meter (Janki Impex Pvt Ltd) etc.
Material and reagent
Reference standard of Paracetamol(gift sample from AUM Research Laboratory, Ahmedabad)and Pamabrom (gift sample from SUVEN Life Science, Hyderabad).HPLC grade methanol from Finar chemicals, Ahmadabad. All other chemicals and reagents used were of AR grade. Mili Q water was use for this study.
Preparation of Standard Solution:
100 mg of Paracetamoland 100mg of Pamabromwas weighedand transferred to a 100ml volumetric flask. About 30ml of diluent was addedand sonicated to dissolve, made up the volume with diluents to get finalconcentration of 1000μg/ml for Paracetamol and 1000μg/ml for Pamabrom. From that 10 ml of PCM and 20 ml of PAM stock solution was withdraw and volume was made upto 100 ml with diluent (Acetonitrile: Phosphate buffer (pH 4.5) (25:75 v/v) which give final concentration of 100 μg/ml and 200 μg/ml respectively.
Selection of analytical wavelength:
The standard solutions of Paracetamol (10 µg/ml) and Pamabrom (10 µg/ml) in methanol were scanned separately in the UV region of 200 to 400 nm and the overlain spectra were recorded as shown in figure 2.
Preparation of calibration curve:
1.0, 2.0, 3.0, 4.0 and 5.0 ml of working standard solution of Paracetamol (100 µg/ml) and 0.75, 1.5, 2.25, 3.0 and 3.75ml of working standard solution of Pamabrom (200 µg/ml) was pipette out and mixed in 10 ml volumetric flask which gives 10-50 µg/ml of PCM and 15-75 µg/ml of PAM. The chromatogram was recorded under the finalized chromatographic conditions as described above after getting a stable baseline. Peak areas were recorded for all the peaks. Calibration curves of Paracetamol and Pamabrom were constructed by plotting the peak area versus concentration (µg/ml) respectively.
VALIDATION PARAMETERS
Parameters such as Linearity and range, Accuracy, Precision, LOD and LOQ were taken up as tests for analytical method validation shown in table 6.
Linearity andRange:
Linearity of the proposed method was verified by analyzing five combined different concentrations in the range of 10-50 μg/ml for Paracetamol and 15-75 μg/ml for Pamabrom. Each concentration was made three times. The calibrationcurve of Peak area versus respective concentration was plotted and regression line equation for Paracetamol and Pamabrom was constructed as shown in figure 6.
Precision
Precision of the method was determined in the terms of Repeatability, Intraday and Interday precision. Repeatability (% RSD) was assessed by analyzing test drug solution within the calibration range, six times on the same day. Intraday variation (% RSD) was determined by analysis of this solution three times on the same day. Interday precision (%RSD) was determined by analysis of this solution on three different days.
Limit of detection (LOD) and limit of quantitation (LOQ)
They were calculated as 3.3 σ/S and 10 σ/S respectively. Where σ is the standard deviation of the response (y-intercept) and S, is the mean of the slope of calibration plot.
Accuracy:
Recovery studies were done so as to check the accuracy of the method. Known amounts of standard solutions of PCM and PAM were added to pre-quantified sample solutions of PCM and PAM and peak area were determined. Concentration of the drug in the sample was calculated using the equations. The analysis was done in a set of 3 replicates at 3 levels (80%, 100% and 120%) as shown in table 3.
Application of Proposed Method to synthetic mixture:
The synthetic mixture of Paracetamol and Pamabrom was prepared in ratio of 13:1. Accurately weighed 325 mg of Paracetamol and 25 mg of Pamabrom were transferred to 100 ml volumetric flask, and 70 ml of methanol was added. Excipients, such as microcrystalline cellulose, povidone, stearic acid, sucroseand corn starch which were used in tablet formulation, were added in this mixture and mechanically stirred for 20 minutes. This solution was filtered through the whatmann filter paper no. 41 and filtrate was collected. Residues were washed with methanol. The filtrate and washing were combined and volume of solution was made up to 100 ml with methanol. 1 ml of this solution was diluted up to 10 ml with diluent. 1.2 ml of this solution was further diluted up to 10 ml with diluent. Chromatogram of the resulting solution was recorded and percentage of label claim was calculated and mentioned in table 4.
RESULTS AND DISCUSSION
RP-HPLC method was analysed at isobestic point 268 nm. To optimize the mobile phase, various proportion of buffer with methanol and acetonitrile were tested. A mobile phase containing mixture of phosphate buffer (PH 4.5):Acetonitrile in the ratio of 75:25 v/v resulted in peaks with good shape and resolution. By applying the proposed method, the retention time for Paracetamol and Pamabrom was found to be 2.665 and 6.907 minutes respectively shown in Figure 3. Peaks were well resolved with resolution of 13.7between the two drugs and were symmetrical in shape with asymmetry factor less than 2.00.Here tailing factor for peaks of Paracetamol and Pamabromwas less than 2. System suitability, robustness study, accuracy and assay were shown in table no: 1,2,3 and 4 respectively.
CONCLUSION
The proposed method was found to be simple, precise, accurate and rapid for determination of Paracetamol and Pamabrom from synthetic mixture. The mobile phase is simple to prepare and economical. The sample recovery in formulation was in good agreement with their respective label claim. Hence, it can be easily and conveniently adopted for routine analysis of Paracetamol and Pamabrom in tablets.
ACKNOWLEDGEMENT
The authors are thankful to Smt. R. D. Gardi B. Pharmacy College, Rajkot, Gujarat, India for providing necessary facilities to carry out this work. We are also thankful to AUM research Lab, Ahmedabad for providing gift sample of Paracetamol and Suven life science, Hyderabad and Pan Drugs Pvt. Ltd., Vadodara for providing the free gift samples of Pamabrom which was required for our research work.
FIGURE AND TABLES
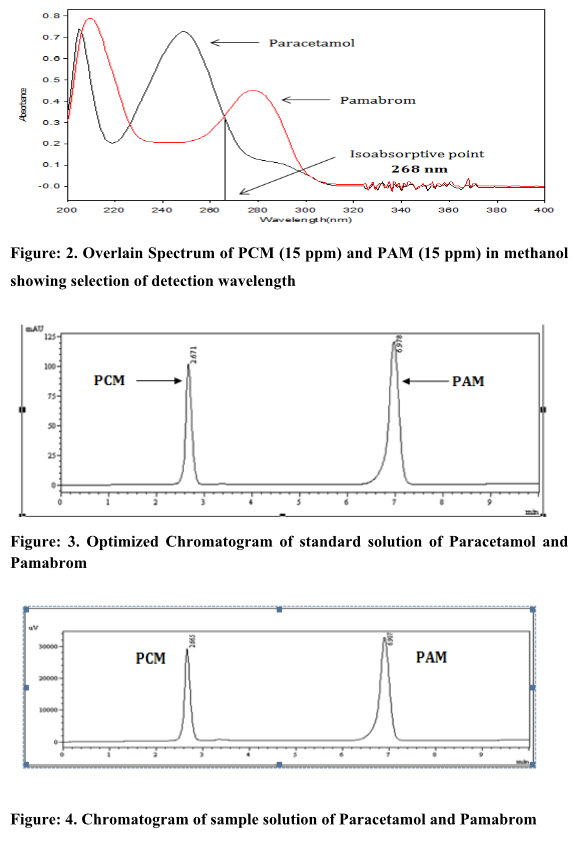
Figure: 2.Overlain Spectrum of PCM(15 ppm) and PAM (15 ppm) in methanol showing selection of detection wavelength
Figure: 3. Optimized Chromatogram of standard solution of Paracetamol and Pamabrom
Figure: 4. Chromatogram of sample solution of Paracetamol and Pamabrom
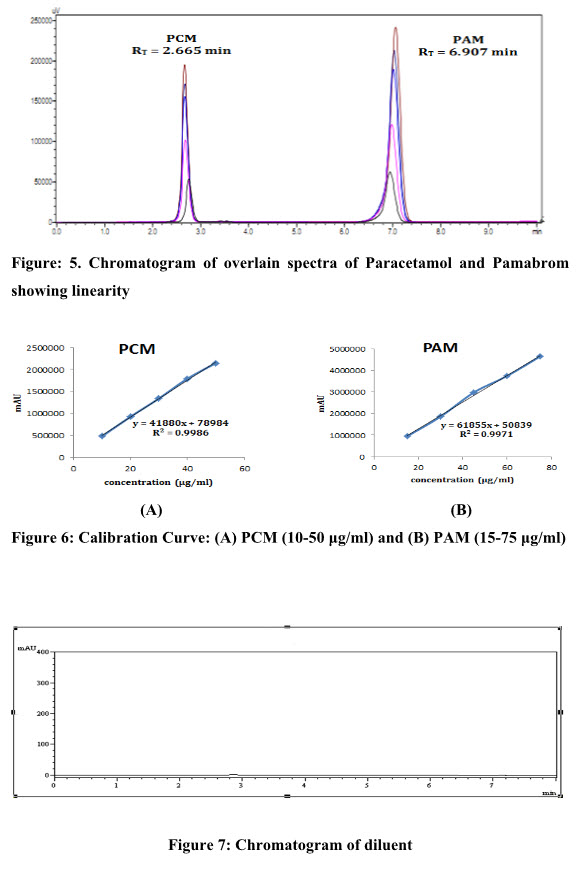
Figure: 5. Chromatogram of overlain spectra of Paracetamol and Pamabrom showing linearity
Figure 6: Calibration Curve: (A) PCM (10-50 μg/ml) and (B) PAM(15-75 μg/ml)
Figure 7: Chromatogram of diluent
Table 1:- System suitability:-
|
Parameter |
PCM |
PAM |
|
Calibration range (μg/ml) |
10-50(μg/ml) |
15-75(μg/ml) |
|
Theoretical Plates |
1978.279 |
5296.496 |
|
Resolution |
13.7 |
|
|
Tailing factor |
1.089 |
0.828 |
Table 2:-Robustness study:-
|
Parameters |
PCM |
PAM |
Rs |
|||
|
RT (min) |
% Recovery |
RT (min) |
% Recovery |
|||
|
Change in flow rate |
0.6 ml/min |
2.668 |
99.67 |
6.985 |
100.67 |
13.945 |
|
1.0 ml/min |
2.662 |
99.75 |
6.978 |
101.05 |
13.824 |
|
|
Change in mob. Phase proportion |
23:77 |
2.601 |
100.21 |
6.914 |
99.97 |
13.452 |
|
27:73 |
2.667 |
101.32 |
6.964 |
98.63 |
13.653 |
|
|
Change in wave- length |
266nm |
2.671 |
100.32 |
6.978 |
99.78 |
13.687 |
|
270nm |
2.671 |
99.95 |
6.978 |
99.65 |
13.995 |
|
Table-3 Result of Recovery Studies for PCM and PAM:
|
Name of Drug |
Amount taken from mixture (μg/ml) |
Amount of Std. drug added (μg/ml) |
Total amount (μg/ml) |
Total amount found (μg/ml) Mean*± SD |
% Mean Recovery |
|
|
20 |
0 |
20 |
19.85 ±0.4294 |
99.25 |
|
PCM |
20 |
16 |
36 |
35.70 ± 0.6244 |
99.16 |
|
20 |
20 |
40 |
39.83 ± 0.6110 |
99.58 |
|
|
20 |
24 |
44 |
44.63 ± 0.8504 |
101.43 |
|
|
|
|
|
|
|
|
|
|
30 |
0 |
30 |
29.45 ±0.6252 |
98.16 |
|
|
30 |
24 |
54 |
53.96 ± 0.7371 |
99.93 |
|
PAM |
30 |
30 |
60 |
60.33 ± 0.9291 |
100.55 |
|
|
30 |
36 |
66 |
67.31 ± 0.4072 |
101.99 |
[*=mean value of 3 determination]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table-4: Analysis of PCM and PAM in synthetic mixture:
|
Synthetic mixture |
Label claim(mg) |
% mean recovery* ± S.D. (% of label claim) |
||
|
PCM |
PAM |
PCM |
PAM |
|
|
325 |
25 |
99.53 ± 0.5890 |
101.93 ± 0.1407 |
|
[*=mean value of 5 determination]
Table-5: Regression Characteristics:
|
Characteristics |
PCM |
PAM |
|
|
|
Linearity (μg/ml) |
10-50 |
15-75 |
||
|
Regression Equation |
y = 41880x + 78984 |
y = 61855x + 50839 |
||
|
Slope |
41880 |
61855 |
||
|
r2 |
0.9986 |
0.9971 |
||
|
Intercept |
78984 |
50839 |
||
|
S.D. of Intercept |
7158.46 |
19058.84 |
||
TABLE-6: VALIDATION PARAMETERS:
|
Parameters |
PCM |
PAM |
|
|
|
Repeatability (%RSD) (n=6) |
0.3849 |
1.0689 |
||
|
Precision (%RSD) |
|
|
||
|
Intra-day (n=3) |
1.0702-1.3660 |
1.2456-1.5787 |
||
|
Inter-day (n=3) |
1.2713-1.7768 |
1.3887-1.7910 |
||
|
LOD (μg/ml) |
0.5640 |
1.0168 |
||
|
LOQ (μg/ml) |
1.7092 |
3.0812 |
||
|
% Recovery (n=3) |
99.16-101.43 |
98.16-101.99 |
||
|
Assay (mean ± S.D.) |
|
|
||
|
(n=5) |
99.53 ± 0.5890 |
101.93 ± 0.1407 |
||
LOD: Limit of Detection, LOQ: Limit of Quantitation, R.S.D.: Relative standard deviation, S.D.: Standard deviation
REFERENCES
1.Sweet man SC., The Martindale: The Complete Drug Reference; 36th Edn; Pharmaceutical Press, London, UK, 2009, pp 978.
2.Rang HP, Dale MM, Ritter JM and Moore PK, Pharmacology, 5th Edn; Elsevier, New Delhi 2006, pp 251,442.
3.The United States Pharmacopoeia-27, NF-22, Asian edition, Rockville, MD: The US Pharmacopoeial Convention, Inc., 2004, pp 1397-98.
4.Drugbase:Drugs?Womankit [Online] 2012 March 30 [cited 2012 Dec 15]; Available from:
URL: drugbase.org/drugs/drug_details.php?drugid=1901
5.Indian Pharmacopoeia, Volume 3, Ghaziabad: Govt. of Indian Ministry of Health and Family Welfare, the Controller of Publication, 2007, The Indian Pharmacopoeial Commission, pp 900-03.
6.British Pharmacopoeia, Volume I-II, London: The Department of Health,: HMSO Publication; 2009, pp 2789
7.Vichare V, Mujgond P, Tambe V and Dhole SN. Simultaneous Spectrophotometric determination of Paracetamol and Caffeine in Tablet formulation. International Journal of PharmTech Research, 2010, 2(4), 2512-2516.
8.Pawar PY, Joshi RS, Jangale KN and Wagh SK. Development and Validation of a Reversed Phase HPLC Method for Simultaneous Estimation of Enalapril maleate, Hydrochlorothiazide and Paracetamol in Pure and its Pharmaceutical Dosage Form. Pelagia Research Library, 2011, 2(5), 121-127.
9.Vyas AJ, Patel JK, Bhandari A, Chavda JR and Sheth NR. SimultaneousEstimation of Lornoxicam and Paracetmaol by Vierodt’s Method in APIand in Synthetic mixture. International Journal of ChemTech Research,2011, 3(3), 1269-1273.
10.ICH guideline Q2 (R1). Validation of analytical procedures: text and methodology. Geneva: 1996.
11.Beckett AH. And Stenlake JB. Practical Pharmaceutical Chemistry, part-II; 4th Edn; CBS Publisher and Distributors, New Delhi, 2007, pp 275-336
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









