ABOUT AUTHORS:
Yogesh Kumar Jain*, Dr. R.P.S. Rathore, Udaibhan Singh Rathore, Dharmendra Singh sisodiya, Vijendra Singh Chauhan
Bhupal Nobles’ College of Pharmacy,
Udaipur – 313002, Rajasthan, India.
*jain.yogesh1987@gmail.com
ABSTRACT
A simple sensitive and precise high performance liquid chromatographic method for the analysis of metronidazole and Norfloxacin has been validated and used for the developed, validated and used for the determination of compound in commercial pharmaceutical products. The compound were well separated on a on hypersil BDS C-18,250×4mm,5µg reversed phase column by use of a mobile phase consisting of mixed phosphate buffers (K2HPO4,KH2PO4)(Ph:6.5) Acetonitrile (55:45 v/v ) at a few rate of 1.0ml min-1 with detection wavelength at 275nm.the linearity range were 5 to 30µg/ml for metronidazole and 0.4-2.4µg/ml for Norfloxacin the recovery amount was more than 99%the high suitability of the method for determination of metronidazole and Norfloxacin in pharmaceutical dose form.
REFERENCE ID: PHARMATUTOR-ART-1831
INTRODUCTION
HPLC is one of the most widely used analytical techniques today, among the different Chromatographic procedure, due to the significant evolution in liquid chromatographic instrument, providing superior qualitative and quantitative results (Inturi et.al., 2011) Method validation is the process used to confirm that the analytical procedure employed for a specific test is suitable for its intended use (Shrivastava et. al.,2011) Metronidazole is used as an antibacterial drug. Chemically metronidazole is as 2-(2-methyl-5-nitro-1H-imidazol-1-yl) ethanol (Dong W et, al., 2009) A survey of literature reveals that HPLC spectrometric method is reported the determination of metronidazole. Norfloxacin is a Synthetic chemotherapeutic antibacterial agent. Chemically it is 1-ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1H-quinoline-3-carboxylic acid .It act by targeting DNA(Basset et. al.,1999).. Analytical methods development and validation play important roles in the discovery, development and manufacture of pharmaceuticals. Literature survey reveals few analytical methods for estimation of metronidazole sodium with other drug combinations (Trinath et. al.,2010). Literature survey revealed that metronidazole and Norfloxacin is official in IP, BP and USP. Although there are many methods reported for estimation of these drugs in combined dosage form. There are different methods reported for quantitative determination of pantaprazole and naproxen bulk or pharmaceutical formulation include titrimetry, colorimetry and high performance liquid chromatography (HPLC)( (Gupta R; USP 2009; Trinath 2010; Haque T 2010;IP 2010; Christen 2001; Ahuja et .al., 2001; Beckett et. al., 1997; Christiana et. al. 1982; Sharma BK et. al., 2005 and Skoog DA et, al, 1994;). The present work was undertaken with an objective to develop an accurate, simple, precise and reliable method for simultaneous estimation of these two drugs in their combined dosage form by HPLC.
MATERIALS AND METHODS
Equipment and chromatographic conditions
The system used consist of SHIMADZU-SPD20AD detector the chromatographic separation was carried out at room temperature with hypersil BDSC-18 reversed phase column by use of a Mobile phase consisting of mixed phosphate buffer (K2HPO4,KH2PO4)(Ph:6.5), acetonitrile (55:45V/V) at a flow rate of 10ml/min. The mobile was filtered through a 0.45µm membrane Filter and degassed for 10mts.The injection volume for samples and standards were 20µg and Eluted at a flow rate of 1ml/min at ambient temperature. The eluents were monitored at 275nm.
Materials and reagents
Metronidazole and Norfloxacin in combination ARTHONF 250(claimed labeled amount 250mg MET and 20mg NF per tablets) was procured from local pharmacies. HPLC grade acetonitrile Was used and all other chemicals (Analytical grade) were used. MET and NF in pure form was Denoted as a gift sample from Madras Pharmaceuticals, Chennai.
Preparation of Standard Solution
A working standard solution containing Metronidazole 250 mg/100ml and Norfloxacin 20 mg/100ml was prepared by dissolving Metronidazole and Norfloxacin sodium reference standard in mobile phase. The mixture was sonicated for 5 minutes or until the reference standard dissolved completely.
Preparation of sample Solution
Twenty tabletss, each containing 250 mg Metronidazole and 20 mg Norfloxacin were accurately weighed and finely powdered. A quantity of powder equivalent to 250 mg of Metronidazole and 20 mg of Norfloxacin was weighted and transferred to a 100 ml volumetric flask. About 70 ml of mobile phase was added and shaken mechanically for 15 minutes. The mixture was then sonicated in ultrasonic bath for 5 minutes and made the volume up to 100 ml by the mobile phase. The solution was filtered with a Whatman filter paper no.1. Before injection, both standard and sample solution was filtered through 0.45 µm syringe filter. Then 10 µl of standard and sample solutions were injected into column and chromatogram was recorded.
Validation of the HPLC method
Linearity
The stock solution of 5-30µg/ml, of MET and 0.4-2.4 µg/ml of NF were prepared and 20 µl fixed volume was injected. Linearity of the method was studied by injecting 6 concentrations of the drug prepared in the mobile phase in triplicate in to the HPLC system keeping the injection volume constant.
Accuracy The accuracy of the analysis was evaluated by determined of recovery at three different Concentrations equivalent to 80,100 and 120% of the amount preanalysed dosage form and average recoveries were calculated.
Precision Five sample of 25mcg were prepared and analyzed as per the sample preparation procedure. System precision and method precision were calculated.
Specificity Specificity studies for method were performed for its ability to asses and unequivocally the MET and NF in the presence of tablets excipients. Chromatographic interferences from tablets excipients were examined. The average retention time for MET and NF were calculated.
Robustness To evaluate robustness of a HPLC method, few parameters were deliberately varied. The parameters included variation of flow rate and the percentage of acetonitrile in the mobile phase.
System Suitability System suitability parameters were evaluated from tailing factor, retention times and theoretical plates of standard chromatograms.
Limit of Detection and Quantitation To determine the LOD and LOQ serial dilutions of mixed standard solutions of MET and NF were made from the standard stock solutions. The samples were injected in HPLC systems on the chromatograms were run and measured signal from the samples was compared with those of blank samples. The limit of detection (LOD) and limit of quantitation (LOQ) were calculated from the slope (s) of the calibration plot and the standard deviations of the response (SD).
RESULTS AND DISCUSSION
The conditions used for chromatography were optimized on the basis of experimentation. The method was validated in accordance with ICH guidelines for linearity, accuracy, precision, specificity and robustness. The mobile phase mixed phosphate buffer: acetonitrile (55:45 v/v) enables good resolution and separation using Hypersil BDS C-18 column (250× 4mm, 5.0µ). The retention time (Rt) values were 3.35min for MET and 4.9min for NF and detectionwavelength 275nm was selected from overlain spectra of the drugs acquired from UV spectrophotometer. [Figure 1]
Linearity
Metronidazole and norfloxacin showed good correlation coefficient (r2 0.999 for NF) in given concentration range 5 and the UV absorbances was taken at 275nm (Table 1 and 2) (Figure 3)
Limit of Detection and Quantification The limit of detection for drugs were found to be 0.158 and 0.026 respectively.
Precision The results of the system precision and method precision experiments are shown in (table 3). The developed method was found to be precise as the %RSD values for system precision and method precision studies were <2%, respectively as recommended by ICH guidelines.
Specificity The method was found to specific as complete separation of both MET and NF in presence of excipients was observed. The average retention time for MET and NF were found to be 3.35min and 4.9min, respectively.
Robustness The robustness of the method was proved by varying the flow rate, mobile phase composition and temperature from the optimized chromatographic conditions and the tailing factors were found to be less than 2%.
Accuracy The proposed method when used for extraction and subsequent estimation of MET and NF from pharmaceutical dosage form after spiking 80,100 and 120% of additional drug afforded average recoveries in between 99.16 to 99.59, for MET and 99.86 to 99.89 for NF. The results indicate that the method enables accurate estimation of the drugs in the tablets dosage form.
CONCLUSION
The proposed HPLC method is simple, accurate and reproducible for simultaneous estimation of metronidazole (MET) and Norfloxacin (NF) in pharmaceutical dosage form, without interference from the excipients. The chromatographic method is validated according to ICH guidelines. Statistical test indicate that the method is suitable for the simultaneous estimation of the above drugs in pharmaceutical dosage form and for routine analysis of raw materials of above drugs in a quality control laboratories, where economy and time are essential.
Table 1: Linearity studies by RP-HPLC
|
Linearity Range |
Stock solution to be taken in mL |
Dilute to volume (mL)with diluent |
Final concentration in µg/mL (Metronidazole) |
Final concentration in µg/mL (Norfloxacin) |
|
25% |
5 |
100 |
10 |
10 |
|
50% |
10 |
100 |
20 |
20 |
|
75% |
15 |
100 |
30 |
30 |
|
100% |
20 |
100 |
40 |
40 |
|
125% |
25 |
100 |
50 |
50 |
|
150% |
30 |
100 |
60 |
60 |
Table 2: Area of Linearity Data
|
Linearity Range |
Area of Norfloxacin |
Area of Metronidazole |
|
25% |
798.74 |
596.04 |
|
50% |
1559.70 |
1164.71 |
|
75% |
2297.95 |
1716.27 |
|
100% |
3059.37 |
2287.05 |
|
125% |
3789.96 |
2833.45 |
|
150% |
4558.59 |
3408.36 |
Table 3: Method Precision Data of Norfloxacin and Metronidazole.
|
Set No. |
% Assay |
% Assay Mean |
%RSD |
|||
|
Metronidazole |
Norfloxacin |
Metronidazole |
Norfloxacin |
Metronidazole |
Norfloxacin |
|
|
1 |
110.5 |
112.1 |
108.4 |
111.6 |
0.7 |
1.1 |
|
2 |
108.7 |
111.2 |
||||
|
3 |
103.5 |
110.3 |
||||
|
4 |
110.4 |
113.4 |
||||
|
5 |
108.1 |
112.3 |
||||
|
6 |
109.4 |
110.4 |
||||
Table 4: Results of Accuracy Data of Norfloxacin
|
For Norfloxacin |
|||||
|
Level |
Amount of Drug added (µg/mL) |
Amount of Drug recovered (µg/mL) |
Recovery (%) |
Mean (%) |
% RSD |
|
80 % |
0.490 |
0.488 |
98.94 |
100.18 |
0.9 |
|
0.490 |
0.491 |
99.47 |
|||
|
0.490 |
0.486 |
102.15 |
|||
|
100 % |
0.900 |
0.992 |
101.15 |
101.44 |
0.2 |
|
0.900 |
0.990 |
101.44 |
|||
|
0.900 |
0.988 |
101.74 |
|||
|
120 % |
1.500 |
1.500 |
100.00 |
99.88 |
0.2 |
|
1.500 |
1.501 |
99.90 |
|||
|
1.500 |
1.489 |
99.75 |
|||
Table 5: Results of Accuracy Data of Metronidazole
|
For Metronidazole |
|||||
|
Level |
Amount of Drug added (µg/mL) |
Amount of Drug recovered (µg/mL) |
Recovery (%) |
Mean (%) |
% RSD |
|
80 % |
3.250 |
3.250 |
100.0 |
100.1 |
0.1 |
|
3.250 |
3.246 |
99.9 |
|||
|
3.250 |
3.250 |
100.0 |
|||
|
100 % |
8.000 |
8.100 |
102.0 |
102.0 |
0.0 |
|
8.000 |
8.100 |
102.0 |
|||
|
8.000 |
8.100 |
102.0 |
|||
|
120 % |
12.500 |
12.475 |
99.8 |
99.8 |
0.0 |
|
12.500 |
12.475 |
99.8 |
|||
|
12.500 |
12.475 |
99.8 |
|||
Figure 1: chemical structure of Metronidazole and Norfloxacine
Figure No 2. Chromatogram of metronidazole and Norfloxacin in acetonitrile buffer, at flow rate 1.0 ml/mnt, at 275nm.
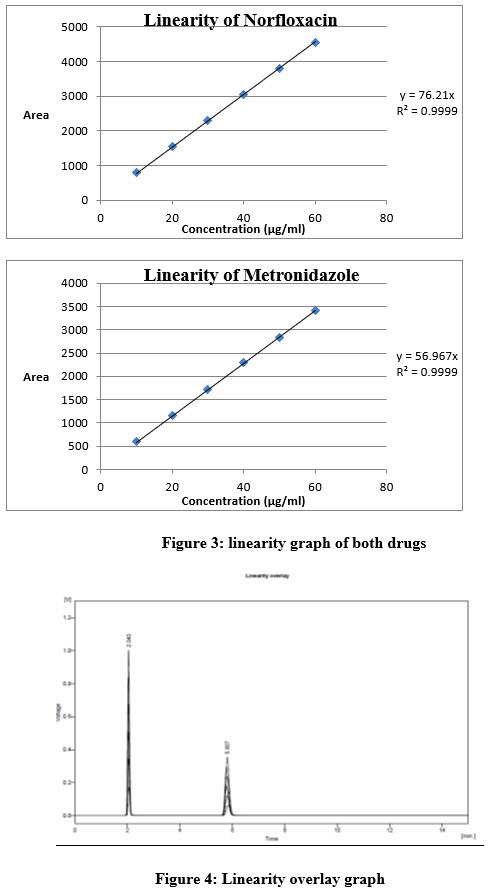
Figure 3: linearity graph of both drugs
Figure 4: Linearity overlay graph
REFERENCES
Ahuja S, Scypinski S. Hand book of modern pharmaceutical analysis, Academic press,USA, 2001,349.
Anand Shrivastava, Anup K Chakraborty, Sujit K Rambhade, Umesh K Patil, Development of validated RP-HPLC method for determination of letrozole in bulk and its pharmaceutical dosage forms Der Pharmacia Sinica, 2011, 2 (2):263-269
Beckett AS,Stenlake JB. Practical pharmaceutical chemistry, 4th edition, CBS publishers and distributors, New Delhi, 1997, 2,278-282
Beckett AS.Stenlake JB, Practical Pharmaceutical chemistry,4th edition,CBS publishers and distributors,New Delhi,1997,2,1-85.
Christen GD, Analytical chemistry,5th edition, John wiley& sons,Inc. New Delhi, 2001, 505
Christianah MA,pui-Kai L, Analytical profile of drug substance, Klaus Florey,New Jersey,1982,11,124-141.
Indian Pharmacopoeia, the Indian Pharmacopoeia Commission, Ghaziabad, 2010, 3, 1745-1755.
Jeffery G.H,Basset J, mendham J,Denney R.C.Vogels.Text book of quantitative analysis, 5th edtion, logmanscientific and technical,1999.10-11
Michael W.Dong, Modern HPLC for practicing scientist. 1st edition, wiley intersciencs, USA, 2009 ,194-,217
Sharma BK, Instrumental method of chemical analysis, 23rd edition, Goel publishing house, Meerut, 2005, 7-8
Skoog DA, West .DM, Holler .FJ. Anaytical chemistry, 6 th edition, Saunder college
Srikanth Inturi, RaviKanth Inturi, G.Venkatesh, Development and validation of analytical method for naproxen and pantoprazole in capsule dosage form, Der Pharmacia Sinica, 2011, 2 (5): 223-234
The United States Pharmacopoeia NF, The official compendia of standards, Rockville, MD, 2009, 3035-3036.
Trinath. M , Saurabh K. Banerjee , Hari Hara Teja. D, Bonde C. G. Development and validation of spectrophotometric method for simultaneous estimation of Sumatriptan and Naproxen sodium in tablet dosage form Der Pharmacia Sinica, 2010, 1 (1): 36-41
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









