About Authors:
Pavitra Solanki1, Satyaveer Singh Solanki2
1- Research Scholar, KIET school of Pharmacy, Ghaziabad.
2- Asstt. Professor, Nehru Mahavidhyalaya, Lalitpur, Uttar Pradesh
*pavitra.bpharma@gmail.com
Abstract
The goal in designing controlled floating delivery system to reduce the frequency of dosing and / or to increase effectiveness of drug by localization at the site of action, reducing the dose required or providing uniform drug delivery with high bioavailability of buoyant drugs and also reduce the severity and frequency of side effects by maintaining patient blood levels of the drug above the minimum effective level and below the minimum toxic level.
Oral route has been the commonly adopted and most convenient route for drug administration. Oral route of administration has been received more attention in the pharmaceutical field because of the more flexibility in the designing of dosage form than drug delivery, design for other routes. For sustained drug delivery dosing interval can be extended either by manipulating the drug molecule to reduce the rate of elimination or by altering the release rate of a dosage form to retard the rate of absorption. Both these approaches decrease fluctuating in plasma level in case of multiple dosing extending the dosing interval without under or over dosing.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1317
1. INTRODUCTION:
The idealized objective of any drug delivery pinpoints two critical aspects of utmost importance i.e. spatial placement and temporal delivery. Controlled drug delivery system provides drug release at a predetermined, predictable and controlled rate to achieve high therapeutic efficiency with minimal toxicity. Despite tremendous advancement in drug delivery, oral route remains the preferred route for the administration of therapeutic agents and oral drug delivery is the most preferable route of drug delivery. The low cost of therapy and ease of administration leads to high levels of patient compliance as well as the fact that gastrointestinal physiology offers more flexibility in dosage form design than most other routes, consequently much effort has been put into development of strategies that could improve patient compliance through oral route.1
One of the most feasible approaches for achieving a prolonged and predictable drug delivery profile in the GI tract is to control the gastric residence time 2,3.Dosage forms that can be retained in the stomach are called gastro retentive drug delivery systems (GRDDS). GRDDS can improve the controlled delivery of drugs that have an absorption window by continuously releasing the drug for a prolonged period of time before it reaches its absorption site thus ensuring its optimal bioavailability 1,2,4, In the proposed work the main objective is to formulate the Buoyant formulation for gastroretantive controlled drug release.
Controlled drug delivery results in optimum therapy, and not only reduces the frequency of dosing, but may also reduce the severity and frequency of side effects by maintaining patient blood levels of the drug above the minimum effective level and below the minimum toxic level.
Gastric emptying of dosage forms is an extremely variable process and ability to prolong and control the emptying time is a valuable asset for dosage forms, which reside in the stomach for a longer period of time than conventional dosage forms. Several difficulties are faced in designing controlled release systems for better absorption and enhanced bioavailability. One of such difficulties is the inability to confine the dosage form in the desired area of the gastrointestinal tract. Drug absorption from the gastrointestinal tract is a complex procedure and is subject to many variables. It is widely acknowledged that the extent of gastrointestinal tract drug absorption is related to contact time with the small intestinal mucosa.27 Thus, small intestinal transit time is an important parameter for drugs that are incompletely absorbed. Basic human physiology with the details of gastric emptying, motility patterns, and physiological and formulation variables affecting the cosmic emptying are summarized.
Gastro retentive systems can remain in the gastric region for several hours and hence significantly prolong the gastric residence time of drugs. Prolonged gastric retention improves bioavailability, reduces drug waste, and improves solubility for drugs that are less soluble in a high pH environment. It has applications also for local drug delivery to the stomach and proximal small intestines. Gastro retention helps to provide better availability of new products with new therapeutic possibilities and substantial benefits for patients.
The controlled gastric retention of solid dosage forms may be achieved by the mechanisms of mucoadhesion,28,29 flotation,30 sedimentation,31,32 expansion,33,34 modified shape systems,35,36 or by the simultaneous administration of pharmacological agents37,38 that delay gastric emptying. Based on these approaches, classification of floating drug delivery systems (FDDS) has been described in detail. In vivo/in vitro evaluation of FDDS has been discussed by scientists to assess the efficiency and application of such systems. Several recent examples have been reported showing the efficiency of such systems for drugs with bioavailability problems.
Gastric residence time of an oral dosage form is affected by several factors. To pass through the pyloric valve into the small intestine the particle size should be in the range of 1 to 2 mm.45 The pH of the stomach in fasting state is ~1.5 to 2.0 and in fed state is 2.0 to 6.0. A large volume of water administered with an oral dosage form raises the pH of stomach contents to 6.0 to 9.0. Stomach doesn’t get time to produce sufficient acid when the liquid empties the stomach; hence generally basic drugs have a better chance of dissolving in fed state than in a fasting state.
[adsense:468x15:2204050025]
Approaches to Design Floating Dosage Forms
The following approaches have been used for the design of floating dosage forms of single- and multiple-unit systems.39
Single-Unit Dosage Forms
In Low-density approach30 the globular shells apparently having lower density than that of gastric fluid can be used as a carrier for drug for its controlled release. A buoyant dosage form can also be obtained by using a fluid-filled system that floats in the stomach.In coated shells40 popcorn, poprice, and polystyrol have been exploited as drug carriers. Sugar polymeric materials such as methacrylic polymer and cellulose acetate phthalate have been used to undercoat these shells. These are further coated with a drug-polymer mixture. The polymer of choice can be either ethylcellulose or hydroxypropyl cellulose depending on the type of release desired. Finally, the product floats on the gastric fluid while releasing the drug gradually over a prolonged duration.
Fluid- filled floating chamber41 type of dosage forms includes incorporation of a gas-filled floatation chamber into a microporous component that houses a drug reservoir. Apertures or openings are present along the top and bottom walls through which the gastrointestinal tract fluid enters to dissolve the drug. The other two walls in contact with the fluid are sealed so that the undissolved drug remains therein. The fluid present could be air, under partial vacuum or any other suitable gas, liquid, or solid having an appropriate specific gravity and an inert behavior. The device is of swallowable size, remains afloat within the stomach for a prolonged time, and after the complete release the shell disintegrates, passes off to the intestine, and is eliminated. Hydrodynamically balanced systems (HBS) are designed to prolong the stay of the dosage form in the gastro intestinal tract and aid in enhancing the absorption. Such systems are best suited for drugs having a better solubility in acidic environment and also for the drugs having specific site of absorption in the upper part of the small intestine. To remain in the stomach for a prolonged period of time the dosage form must have a bulk density of less than 1. It should stay in the stomach, maintain its structural integrity, and release drug constantly from the dosage form. The success of HBS capsule as a better system is best exemplified with chlordiazeopoxide hydrochloride. The drug is a classical example of a solubility problem wherein it exhibits a 4000-fold difference in solubility going from pH 3 to 6 (the solubility of chlordiazepoxide hydrochloride is 150 mg/mL and is ~0.1 mg/mL at neutral pH).
HBS of chlordiazeopoxide hydrochloride42 had comparable blood level time profile as of three 10-mg commercial capsules. HBS can either be formulated as a floating tablet or capsule. Many polymers and polymer combinations with wet granulation as a manufacturing technique have been explored to yield floatable tablets.
Various types of tablets (bilayered and matrix) have been shown to have floatable characteristics. Some of the polymers used are hydroxypropyl cellulose, hydroxypropyl methylcellulose, crosspovidone, sodium carboxymethyl cellulose, and ethyl cellulose. Self-correcting floatable asymmetric configuration drug delivery system39employs a disproportionate 3-layer matrix technology to control drug release.
The 3-layer principle has been improved by development of an asymmetric configuration drug delivery system in order to modulate the release extent and achieve zero-order release kinetics by initially maintaining a constant area at the diffusing front with subsequent dissolution/erosion toward the completion of the release process. The system was designed in such a manner that it floated to prolong gastric residence time in vivo, resulting in longer total transit time within the gastrointestinal tract environment with maximum absorptive capacity and consequently greater bioavailability. This particular characteristic would be applicable to drugs that have pH-dependent solubility, a narrow window of absorption, and are absorbed by active transport from either the proximal or distal portion of the small intestine.
Multiple-Unit Dosage Forms
The purpose of designing multiple-unit dosage form is to develop a reliable formulation that has all the advantages of a single-unit form and also is devoid of any of the above mentioned disadvantages of single-unit formulations. In pursuit of this endeavor many multiple-unit floatable dosage forms have been designed. Microspheres have high loading capacity and many polymers have been used such as albumin, gelatin, starch, polymethacrylate, polyacrylamine, and polyalkylcyanoacrylate. Spherical polymeric microsponges, also referred to as “microballoons,” have been prepared. Microspheres have a characteristic internal hollow structure and show an excellent in vitro floatability.43 In Carbon dioxide–generating multiple-unit oral formulations44several devices with features that extend, unfold, or are inflated by carbon dioxide generated in the devices after administration have been described in the recent patent literature. These dosage forms are excluded from the passage of the pyloric sphincter if a diameter of ~12 to 18 mm in their expanded state is exceeded.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Classification of Floating Drug Delivery Systems (FDDS)
Floating drug delivery systems are classified depending on the use of 2 formulation variables: effervescent and non-effervescent systems.
Effervescent Floating Dosage Forms
These are matrix types of systems prepared with the help of swell able polymers such as methylcellulose and chitosan and various effervescent compounds, e.g., sodium bicarbonate, tartaric acid, and citric acid. They are formulated in such a way that when in contact with the acidic gastric contents, CO2 is liberated and gets entrapped in swollen hydrocolloids, which provides buoyancy to the dosage forms.
Ichikawa et al44 developed a new multiple type of floating dosage system composed of effervescent layers and swell able membrane layers coated on sustained release pills. The inner layer of effervescent agents containing sodium bicarbonate and tartaric acid was divided into 2 sub layers to avoid direct contact between the 2 agents. These sub layers were surrounded by a swell able polymer membrane containing polyvinyl acetate and purified shellac. When this system was immersed in the buffer at 37ºC, it settled down and the solution permeated into the effervescent layer through the outer swell able membrane. CO2 was generated by the neutralization reaction between the 2 effervescent agents, producing swollen pills (like balloons) with a density less than 1.0 g/ml. It was found that the system had good floating ability independent of pH and viscosity and the drug (Para-amino benzoic acid) released in a sustained manner28 (Figure 2, A and B).
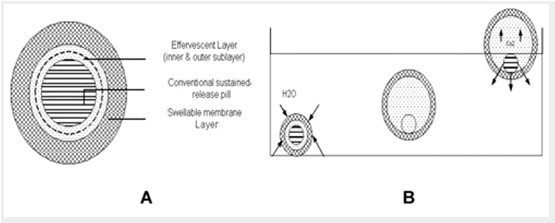
Figure 2. (A) Multiple-unit oral floating drug delivery system. (B) Working principle of effervescent floating drug delivery system.
INVESTIGATION:
Conventional drug delivery system is associated with certain drawbacks. In which three – four times a day drug should given to a patient, which is non-compliance to patients so it will enhance the cost of therapy and bioavailability of the drug. The developments of buoyant formulation are to be preferred over single unit dosage forms (SUDs) because of various advantages.
As sustained release systems, floating dosage forms offer various potential advantages evident from several recent publications. Drugs that have poor bioavailability because of their absorption is restricted to the upper GI tract can be delivered efficiently thereby maximizing their absorption and improving their absolute bioavailabilities. For instance, a significant increase in the absolute bioavailability of the floating dosage form of furosemide has been obtained (42.9%), compared to the commercially available formulation (Lasix®; 33.4%) and enteric product (Lasix® long; 29.5%). Furthermore, among these three dosage forms, only the floating dosage form yielded satisfactory in-vitro results that were significantly correlated (P<0.05) with in vivo absorption kinetics. The findings of this study were based on a previous postulation that site-specific absorption and longer GRT could possibly increase the bioavailability of furosemide26.
5. MATERIAL AND METHODS:
Materials: Drugs, Polymers, floating Polymers, Diluents, Lubricants, Colouring agents, organoleptic additives, etc.
Instruments:Digital weighing balance, Planetary mixer, Fluidized bed dryer, Compression machine, Friabilator, Hardness tester, Moisture content analyzer, Tapped density / Bulk density Apparatus, pH meter, U.V. spectrophotometer ,Six station dissolution rate apparatus ,Humidity chamber, Mechanical stirrer , Centrifuge, H.P.L.C., etc.
The buoyant tablet will be formulated by wet granulation, dry granulation and direct compression technique. In wet granulation technique aqueous and non aqueous granulation will be using. Finally compressed tablet will be coated by aqueous and non aqueous enteric and sugar coating. After that packing material compatibility with tablet will be check and finally drug stability as per ICH guideline.
Formulation Trials
* Various formulations will be planned by using statistical tools like Design Of Experiments (DOE) which will optimize the quantity of various Excipients to be used to design 2 hours, 4 hours, 6 hours, 8 hours, 10 hours and 12 hours controlled buoyant dosage form.
* Evaluation of Formulation Trials
v The different time release dosage forms will be analysed for
§ Content Uniformity ( assay of 6 units )
§ Assay
§ Floating time
§ Floating duration
§ Friability (using friability tester)
§ Dissolution testing ( using USP type IV apparatus)
* Stability Study Data Presentation
v The most appropriate formulation will be kept on stability station as follows:
|
S.No. |
Station |
Time period |
|
1. |
25°c – 60%RH |
15 days ,1, months |
|
2. |
30°c – 65%RH |
15 days ,1, months |
|
3. |
40°c – 75%RH |
15 days ,1, months |
|
4. |
Long term study |
_____ |
7. SUMMARY:
Drug absorption in the gastrointestinal tract is a highly variable procedure and prolonging gastric retention of the dosage form extends the time for drug absorption. FDDS promises to be a potential approach for gastric retention. Although there are number of difficulties to be worked out to achieve prolonged gastric retention, a large number of companies are focusing toward commercializing this technique.
The purpose of writing this review on floating drug delivery systems (FDDS) was to compile the recent literature with special focus on the principal mechanism of floatation to achieve gastric retention. The recent developments of FDDS including the physiological and formulation variables affecting gastric retention, approaches to design single-unit and multiple-unit floating systems, and their classification and formulation aspects are covered in detail. This review also summarizes the in vitro techniques, in vivo studies to evaluate the performance and application of floating systems, and applications of these systems. These systems are useful to several problems encountered during the development of a pharmaceutical dosage form.
8. REFERENCES:
1. Chawla G., Gupta P., Koradia V., Bansal, A.K.,Gastroretention- a means to addressregional variability in intestinal drug absorption. Pharm. Tech. 2003, 27(7): 50-51.
2. Vyas, S.P., Khar.R.K.,Targeted and Controlled Drug Delivery Novel Carrier System, Ist Ed., CBS Publishers and Distributors, New Delhi, 2000, pp.417-454.
3. Singh, B.N., Kim, K.H.,Floating drug delivery systems: an approach to oral controlleddrug delivery via gastric retention. J. Controlled Release. 2000, 63 (1-2): 235-259.
4. Garg, S., Sharma, S.,Gastroretentive drug delivery systems. Pharm. Techh.2003, 13(1): 160.
5. Dennis A, Timminis P, Lel K. Buoyant controlled release powder formulation. US patent 5, 1992,169,638
6. Timmermans, J., Moes, A.J.,Factors controlling the buoyancy and gastric retentioncapabilities of floating matrix capsules, J. Pharm. Sci. 1994,83 (1): 18-24.
7. Aldrete M.E., Villfuence R.L.,Influence of the viscosity and the oarticle size of HPMC onmetronidazole release from matrix tablets, Eur. J. Pharm. Biopharm. 1997,43: 173-178.
8. Whitehead, L., Fell, J.T., Collett, J.H.,Development of a gastroretentive dosage forms,Eur. J. Pharm. Sci. 1998, 4 (1): S-182.
9. Katayama, H., Nishimura, T., Ochi, S., Tsuruta, Y., Yamazaki, Y.,Sustained release liquid preparation using sodium alginate for eradication of Helicobacter pylori. Biol. Pharm. Bull.1999, 22:55-60.
10. Baumgartner, S., Kristi, J., Vrecer, F., Vodo pivec, P., Zorko, B.,Optimisation of floating matrix tablets and evaluation of gastric residence time. Int. J. Pharm.2000, 195 (1):125-135.
11. Shoufeng Li, Senshang Lin, Yie, W.Chien, Daggy P. Bruce.,Statistical optimization of gastric floating system for oral controlled delivery of calcium. AAPS Pharm. Sci. Tech. 2000, 2(1): 1-5.
12. Wong P.S.L., Dong L.C., Edgren D.E., Theeuwes F.,Prolonged release active agent dosage form adapted for gastric retention. US patent 6, 2000,120 803.
13. Chen Jun, Blevins W.E.,Gastric retention properties of superporous hydrogel composites. J. Control. Rel.2000, 64: 39-51.
14. Nur A. O., Zhang J. S.,Captopril floating and/or bioadhesive tablets: design and release kinetics. Drug Dev. Ind. Pharm. 2000, 26: 965- 969.
15. Whitehead Lynne,Amoxycillin release from a floating dosage form based on Alginates, International Journal of Pharmaceutics. 2000, 210: 45- 49.
16. Asmussen, B., Cremer, K., Hoffmann, H.R., Ludwig, K., Roreger, M. Expandable gastroretentive therapeutic system with controlled active substance release in gastrointestinal tract. US patent 6, 2001,290 989.
17. Sato, Y., Kawashima, Y., Takeuchi, H., Yumato, Y., Fyibayashi, Y.,Pharmacoscintigraphic evaluation of riboflavin-containing floating micro balloons for floating controlled drug delivery system in healthy human volunteers. J.Microencapsulation. 2001, 18 (1): 65-75.
18. Lee, J.H., Park, T.G., Choi, H.K.,Development of oral drug delivery system using floating microspheres, J. Microencapsulation. 2002, 16 (6): 715-729.
19. Innaucelli., Floating microspheres of Eudragit, The AAPS Journal.2002, E22.
20. Kleusener, E.A., Lavy, E., Stepensky, D.,Furosemide pharmacokinetics and pharmacodynamics following gastroretentive dosage form administration to healthy volunteers. J. Clin. Pharmacol. 2003, 43: 711-720.
21. Basak, S.C., Nageswara Rao, K.,Development and in vitro evaluation of an oral floating matrix tablet formulation of ciprofloxacin. Indian J. Pharm. Sci. 2003, 65 (3): 313-316.
22. Streubel A. Siepmann J.,Floating matrix based on low-density foam powder: effects of formulation and processing parameters on drug release. Eur. J. Pharm. Sci. 2003, 18: 37-45.
23. Dave, B.S., Amin, A.F., Patel, M.M.,Gastroretentive drug delivery system of ranitidine hydrochloride. AAPS. Pharm. Sci. Tech. 2004, 5(2): 1-6.
24. Pattnaik, S., Suresh, P.,Design and evaluation of gastro retentive drug delivery system. International Convention of APTI, 2004, pp 33.
25. Yiqiao, H., Feng, Z., Minjie, S.,Xiaoqiang, X.,Floating Matrix dosage form for phenoporlamine hydrochloride based on gas forming agent:In vitro and In vivo evaluation in healthy volunteers.International Journal of Pharmaceutics, 310(2006) 139-145.
26. Ritschel, W.A. Menon, A., Sakr, A., Biopharmaceutic evaluation of furosemide as a potential candidate for a modified release peroral dosage form, Methods Find. Exp. Clin. Pharmacol. 1991, 13: 629–636.
27. Hirtz J. The git absorption of drugs in man: a review of current concepts and methods of investigation. Br J Clin Pharmacol. 1985;19:77S-83S.
28. Ponchel G, Irache JM. Specific and non-specific bioadhesive particulate system for oral delivery to the gastrointestinal tract. Adv Drug Del Rev. 1998;34:191-219.
29. Lenaerts VM, Gurny R. Gastrointestinal Tract- Physiological variables affecting the performance of oral sustained release dosage forms. In: Lenaerts V, Gurny R, eds. Bioadhesive Drug Delivery System. Boca Raton, FL: CRC Press; 1990.
30. Deshpande AA, Shah NH, Rhodes CT, Malick W. Development of a novel controlled release system for gastric retention. Pharm Res. 1997;14:815-819.
31. Red patent 3 507 952. April 22, 1970. nick AB, Tucker SJ, inventors. Sustained release bolus for animal husbandry. US
32. Davis SS, Stockwell AF, Taylor MJ, et al. The effect of density on the gastric emptying of single and multiple unit dosage forms. Pharm Res. 1986;3:208-213.
33. Urguhart J, Theeuwes F, inventors. Drug delivery system comprising a reservoir containing a plurality of tiny pills. US patent 4, 434 153. February 28, 1994.
34. Mamajek RC, Moyer ES, inventors. Drug dispensing device and method. US Patent 4 207 890. June 17, 1980.
35. Fix JA, Cargill R, Engle K. Controlled gastric emptying. III. Gastric residence time of a non-disintegrating geometric shape in human volunteers. Pharm Res. 1993;10:1087 1089.
36. Kedzierewicz F, Thouvenot P, Lemut J, Etienne A, Hoffman M, Maincent P. Evaluatio of peroral silicone dosage forms in humans by gamma-scintigraphy. J Control Release. 1999;58:195-205.
37. Groning R, Heun G. Oral dosage forms with controlled gastrointestinal transit. Drug Dev Ind Pharm. 1984;10:527-539.
38. Groning R, Heun G. Dosage forms with controlled gastrointestinal passage—studies on the absorption of nitrofurantion. Int J Pharm. 1989;56:111-116.
39. Yang L, Fassihi R. Zero order release kinetics from self correcting floatable configuration drug delivery system. J Pharm Sci. 1996;85:170-173.
40. Burns SJ, Attwood D, Barnwell SG. Assesment of a dissolution vessel designed for use with floating and erodible dosage forms. Int J Pharm. 1998;160:213-218.
41. Joseph NJ, Laxmi S, Jayakrishnan A. A floating type oral dosage from for piroxicam based on hollow microspheres: invitro and in vivo evaluation in rabbits. J Control Release. 2002;79:71-79.
42. Sheth PR, Tossounian JL, inventors. Sustained release pharmaceutical capsules. US patent 4,126,672. November 21, 1978.
43. Soppimath KS, Kulkarni AR, Rudzinski WE, Aminabhavi TM. Microspheres as floating drug delivery system to increase the gastric residence of drugs. Drug Metab Rev. 2001;33:149-160.
44. Ichikawa M, Watanabe S, Miyake Y. A new multiple unit oral floating dosage system. I: Prepration and in vitro evaluation of floating and sustained-release kinetics. J Pharm Sci. 1991; 80:1062-1066.
45. Wilson CG, Washington N. The stomach: its role in oral drug delivery. In: Rubinstein MH, ed. Physiological Pharmacetical: Biological Barriers to Drug Absorption. Chichester, UK: Ellis Horwood; 1989:47-70.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









