 About Author:
About Author:
Maanasa.B,
Sri Sai Aditya Pharmaceutical Sciences & Research,
Andhrapradesh
ABSTRACT:
Biomarkers in general used as an indicator of the biological response, pathological response and pharmacological responses and mostly used in scientific fields. Specifically, biomarkers indicate change in expression or the protein nature that correlate with disease progression. An ideal biomarker must be sensitive, specific and accurate and must be able to reveal the nature of disease / able to predict the outcome of the tumor reoccurrence. There are different types of biomarkers individualizing different roles like diagnostic biomarkers, detection biomarkers, prognostic biomarkers, predictive biomarkers. These biomarkers are used throughout the clinical trials process of cardiology, neurology and oncology and the present article describes the biomarkers.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1231
INTRODUCTION:
Biomarkers are the characteristic that judges the process normal/abnormal. It is a pharmacological retort that indicates the efficacy of a specific drug, or a biological molecule that designate the condition of the ailment state.Biomarkers are used both in clinical development and medical practice.
Biomarkers in clinical practice
* Supports more capable research and development methods
* Identifies risk groups
* Identifies peril of toxicity
Biomarkers in medical development
* Addresses ailments of aging inhabitants
* Evaluate disease risk
* Assists diagnose patients before time in disease maturity to diminish health care outlay
* Provides predictive tools
AIM & SCOPE:
*Biomarkers for clinical safety assessment .
*Clinical pharmacogenomics. Biomarkers to monitor and predict disease progression and response to therapy.
*Clinical validation of biomarkers.
*Biomarkers as indications for drug therapy.
*Biomarker assay development and validation.
*Outcome diagnostics. Cancer diagnostics.
*Monitoring response to therapy. Discovery-to-Diagnostic translation.
*Expression signatures as diagnostic/prognostic tools.
Supreme Marker for diagnosis:
– ought to have sensitivity, specificity, and accuracy in reflecting total disease. A tumor marker should also be predictive of result and of tumor return and success of anti-cancer treatment.
– The marker must be able to clearly reflect the early stage of disease
– The method for screening should be cost effective
DETECTION OF BIOMARKER:
Biomarkers are detected in two ways both quantitative and qualitative. Quantitative a link between quantity of the marker and disease .Qualitative a link between exist of a marker and disease.
SAMPLES FOR BIOMARKERS: Blood, urine samples and other body fluid and tissue samples are used.
APPLICATIONS OF BIOMARKERS:
*In the field of Oncology Breast cancer, Pancreatic Cancer, Prostate Cancer ,Lung Cancer, Gastric Cancer.
* Clinical Laboratory.
* Decision making on early drug development.
* Measurement in biological samples (plasma, serum ,CSF).
* Clinical trials in systematic sclerosis ,Diabetes, Alzheimer's disease.
* In the field of Cardio Vascular disease (CVD).
Sprouting role of biomarkers:
Biomarker science progress faster than acceptance-
Biomarkers assists in evaluation of the disease characteristics like cardiovascular risk can be estimated through blood pressure, prostate specific antigen assess the prostate cancer risk and grade. Prostate specific antigen (PSA) has taken decades to evolve as a biomarker.
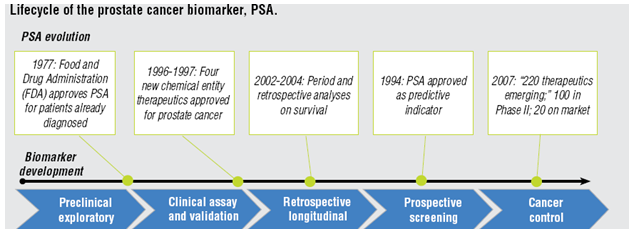
Common biomarkers in cancer
AFP (>100ng/ml): alpha fetoprotein
CEA(>10ng/ml): carcinoembryonic antigen
CA19-9(>37U/ml): carbohydrate antigen
hTG(>10ng/ml): thyroglobulin
HCG(>10mlU/ml): human chorionic gonadotropin – beta
BIOMARKERS IN ALZHEIMER’S DISEASE:
One of the troubles of Alzheimer's disease is that symptoms of disease emerge to widen only after substantial cell loss has occurred in brain. Successful biomarker tests could avert such devastating smash up occurring. This will be predominantly significant once as a heal or more valuable medications become available.
[adsense:468x15:2204050025]
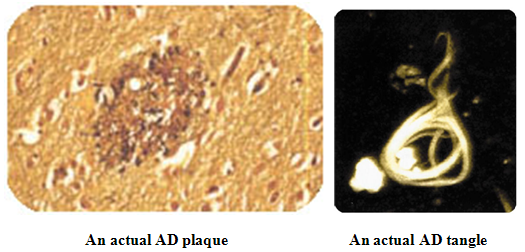
Current Biomarkers for Alzheimer's disease
Current biomarkers for Alzheimer's disease include:
*Beta-amyloid calculated in cerebrospinal fluid
*Tau protein considered in cerebrospinal fluid
*Neural thread protein/AD7C-NTP measured in cerebrospinal fluid and in urine.
Selected Companies Working in Biomarker-Related Drug R&D:
Affymetrix , Inc. Agendia B.V. Agilent Technologies, Inc., LSCA group Applied Biosystems BioSite , Inc. Caliper Life Sciences, Inc. Celera Group Cepheid CombiMatrix Corp (Acacia Research) Dako A/S diaDexus , Inc. Epigenomics AG Fluidigm Corporation Gene Logic, Inc. NimbleGen Systems, Inc. Nymox Pharmaceutical Corporation Orion Genomics LLC Qiagen NV Roche Molecular Diagnostics GVK-BIO.
CONCLUSION:
• They help in finding new targets to alleviate tumor.
• They laid a innovative way in early verdict of cancer.
• Investigations has to be done, to widen bio-markers as consistent indicative and remedial objective.
REFERENCE:
1. seminarprojects.com/Thread-bio-markers-in-cancer-diagnosis-and-treatment#ixzz1k74jIjHd
2. biomarker.co.uk/what-is-a-biomarker.html
3. Biomarkers for Psychiatric Disorders. Publisher: Springer U.S. DOI: 10.1007/978-0-387-79251-4 Copyright: 2009 ISBN 978-0-387-79250-7 (Print) 978-0-387-79251-4 (Online)
4. Smith, Sorensen, and Thrall,Biomarkers in Imaging: Realizing Radiology’s Future; Radiol 2003; 227:633-638
5. Meschan, Farrer-Meschan, Roentgen Signs in Clinical Diagnosis, W.B. Saunders Company, Philadelphia, 1956
6. Smith JJ, Shyjan AM. Defining “least burdensome means” under the Food and Drug Administration Modernization Act of 1997. Food Drug Law J 2000; 55:435-447
7. Goodsaid, Frueh. Biomarker Qualification Pilot Process at the US FDA. AAPS Journal. 2007; 9(1):105-108
8. Schatzkin A, Gail M. The promise and peril of surrogate endpoints in cancer research. Nat Rev Cancer 2002; 2:19-27
9. Gove PB, ed. Webster’s Seventh New Collegiate Dictionary. Springfield, MA:G & C Merriam Company;963:282.
10. List of drugs Courtney claims to have diluted [press release]. Kansas City, MO:Kansas City Division, US Department of Justice Federal Bureau of Investigation; April 22, 2002. Availableat: kansascity.fbi.gov/kcmostate042202 htm. Accessed July 6, 2002.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









