 ABOUT AUTHOR
ABOUT AUTHOR
Vinay Kumar Singh.
Chief Research Officer
Parammount Cosmetics India Limited,
Bangalor, Karnataka
vinay@parammount.com
Product is successful when it sells in the market and brings profit and revenue for the manufacturer. For a successful product, quality is an important factor for consumer to accept it. In order to ascertain quality of a product, it must be tested on various parameters. Without testing the product, one can not be sure of its quality, safety and efficacy. Before any new product is put in the market, it must be thoroughly tested.
[adsense:336x280:8701650588]
Product testing program involves following:
a) Stability Testing
b) Safety testing
c) Performance/Efficacy testing
Stability testing
Stability testing evaluates a product’s ability to maintain its original aesthetic, physical and chemical characteristics under controlled conditions
designed to accelerate aging. Such testing can provide an early indication of problems that may occur in formulations.
Why stability testing is done?
Stability data is useful as an “early warning system” that alerts a formulator about problems related to formulation, Package etc.
Such advance information can be helpful in many ways: -
• To guide the chemist during product development
• To ensure that the product will continue to be aesthetically acceptable to the consumer.
• To determine that the product will perform as intended and remain safe to use.
• To forewarn the manufacturer about problems which may occur after the consumer has purchased the product.
Thus, stability testing gives us an idea of the future risks and provides us guidelines to lay down a foundation for evaluation of future problems.
When stability testing is done?
Stability testing is done:
• Whenever any new formulation is developed.
• Qualifying new raw material
• A modification in the formula
• A modification in manufacturing process
• Change in immediate packaging.
How Stability testing is done?
Stability testing involves:
• Testing of formulation in initial stages of product development.
• Compatibility testing.
Changes that occur in the product over a period of time includes following:
a) Physical
- Viscosity
- Texture
- Colour
- Odour
- Loss of Volatile constituents
- Uptake of water
b) Chemical
- pH
- Degradation of active constituents
- Interaction between constituents
c) Microbiological Spoilage
Changes that occur in the container over a period of time includes following: - Leakage
- Corrosion
- Stress cracking
To obtain the stability data in short time accelerated stability testing is done.
Accelerated stability testing involves exposure of the product to following parameters:
• Elevated temperature
• Elevated humidity
• Cycling tests
• Freeze-thaw tests
• Exposure to light
• Mechanical tests.
(1) Elevated temperature:
Storage at elevated temperature is critical, since the rate of chemical reaction roughly doubles for every ten degree increase in temperature. This test allows us to see certain problems much sooner than they would appear at room temperature. The drawback of this process is that, at high temperature we may be forcing reactions to occur that would not happen at all at lower temperatures.
The most common storage conditions includes 45oC, 54oC, 37oC, 35oC, room temperature, 4oC.
It is recommended to store enough sample to make all the observations required as per your specification.
At each checkpoint product should be checked for following parameters
a) Physical attributes such as colour, odour, viscosity etc.
b) Microbial Challenge test for preservative efficacy
c) Percentage of actives
d) Functional attributes
(2) Elevated Humidity
Since many products are adversely affected by moisture, storage at elevated humidity normally forms part of stability tests.
(3) Freeze – thaw tests Subjecting a product to alternate freezing and thawing can be of value in indicating the tendency of liquid products to cloud or crystallize or the physical stability of creams or other liquid of creams or other liquid emulsions.
(4) Cycling tests
Tests under conditions that are periodically changed can impose greater stress on samples than storage under constant conditions. The following are suggested as generally useful cycling conditions.
• 370c / 80% r.h alternating 24 hourly with 200c /ambient humidity.
• Mean maximum temperature
• Mean maximum humidity alternating 24 hourly with 200c /ambient humidity.
(5) Exposure to sunlight
Where products are likely to be exposed to light in the market or in use, it is necessary to investigate the effect of such exposure. Mostly the effect of sunlight is seen as change in colour of the products.
(6) Mechanical tests
Vibration of samples can be useful in indicating whether demixing is likely to occur in powder or granular products; it can also serve as an indicator of emulsion stability. Clear definition of test objectives and careful planning of tests can yield the required information most efficiently and in the shortest time.
Compatibility testing goes hand in hand with stability testing. This test highlights the interaction between contents & the immediate container.
These interactions may be of following types
• Sorption of constituents of the contents by the container.
• Leaching of constituents of the container into the contents.
• Adverse effects on the container such as corrosion
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Safety Testing
The cosmetic product regulation of the consumer protection Act says that all cosmetics & toiletries shall not harm their users. All the products must be formulated keeping in mind the safety of end-user.
Safety testing programme involves 3 tests: -
1) Microbiological Safety Tests
2) Toxicity Tests
3) Irritation and sensitization Tests.
(1) Microbiological Safety Tests
• Bacteria, moulds and yeasts are everywhere. Most cosmetic products particularly those with a water content are potentially food and a growing place for micro-organisms. If they gain a foothold in a product they will quite likely spoil it and might even present a health hazard to the user.
• Because no one wants to keep refrigerated their cosmetics and use within 2 days of purchase like food, most cosmetic products will require the addition of preservative. Its function is to inhibit growth of micro-organisms in the product. It is therefore a poison, so its proportion in the product needs to be small as possible.One must ensure that the freshly manufactured product is as free from micro-organisms as possible. The preservative will have enough to do killing all those which gain entry during the use of the product from fingers, from lips and from left-off tops.
• Both raw materials & finished products are checked for microbial content.This is done by diluting a sample of known volume with diluent and using this to inoculate an agar jelly plate. This is incubated at 37oC for 48 hours. This incubation period is enough for each viable micro-organism to grow into a colony large enough to be seen and counted. From this count the number of viable micro-organisms in each cm3 of the product can be calculated. This is the total viable count or TVC. The aim should be a TVC of less than 10 per cm3 .
A manufacturer not only wants to know if his products are of good microbiological quality but also that the preservatives he had added will be effective. To check this, Microbial Challenge test is performed.
(2) Toxicity Tests
A toxic substance is a poisonous substance. As far as possible, no toxic materials should be used in cosmetics. However there are certain materials which at a low percentage are safe to use but at high conc. are toxic. Each countries legislation lays down maximum limits on the proportion of these materials. For eg:- Formaldehyde in nail hardners : max-5%, Asbetos in talc is not allowed as it is carcinogenic, Boric acid in talcum powders :Max – 5%(Must state: ‘ not to be used for children under 3 years of age’) For cosmetic purpose it is essential to use materials of a suitable standard of purity. All raw materials intended for use in consumer products have been tested for potential toxicity. Batches of laboratory animals (rats) are given a dose of test substance in proportion to the level of the substances in the finished product to see if it causes death or harm. The results enable the legislation to state whether a material may be used freely or be tightly controlled or to be banned.
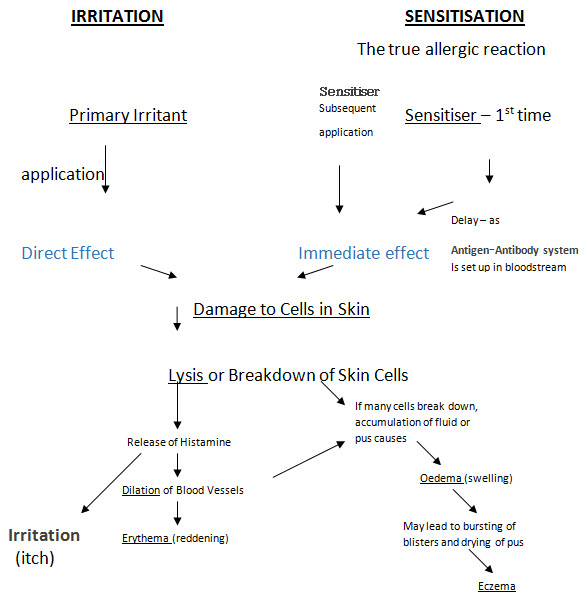
3)Irritation & Sensitization Testing
Allergic reactions- There are many substances which to the majority of the users are completely safe but to a few they produce allergies. These allergic reactions are of 2 types. - Primary Irritation & Sensitization Irritants & sensitizers in cosmetics: Some known Irritants & sensitizers are quite widely used in cosmetics due to following reasons:-
(1) Unavailability of safer alternative Eg: para-phenylene diamine & para-tolydene diamine in hair dyes. Thioglycollic acid in perm lotion.
(2) a substance is so valuable as to make its use worth ( the risk involved in it may adversely affect only very few people).Eg:- Lanolin – It is an excellent emollient and replacement for sebum. Some people are allergic to it.
When a known irritant or sensitizer is included in a product, it must be stated on the label, together with any special instructions.For eg: - Contains phenylene diamine. Skintest advised. Discontinue use if irritation occurs.Do not use on broken skin etc.
The Mechanism of Skin Irritation and Sensitization
Testing for irritation and sensitization :-
• Patch Testing
• Photosensitivity
• Eye irritation testing
• Inhalation testing
• Acnegenicity
• Systemic effects
Patch Testing
This involves following steps
1.Preparing a patch test strip
2.Applying the samples to the patches
3.The patch test strip is placed on the arm or back for 48 hours.
4.The patches are removed after 48 hrs
5.The skin is inspected for signs of irritation, reddening, itching or blistering, etc.
The mechanism of irritation: -
An irritant substance cause following sequence of symptoms
1. Irritation – itch or pain
2. Erythema – reddening
3. Oedema - Swelling, blistering
4. Eczema- blisters burst
The symptoms will depend on Conc. of irritant and Sensitivity of person’s skin to it.
Photosensitivity
Some substances only produce adverse reactions when exposed to light. These are called as phototoxic or photo allergenic materials.
In this case also, patch test is carried out and the subject is exposed to UV radiation regularly throughout a 3- week period and again after 14day rest period with one single exposure. They are then evaluated for allergic response. Any phototoxic effects generally appear within 24 hrs and subside after 72 hrs. The effects of phototoxic substances includes intense erythema, hyper pigmentation, sunburn, edema & blistering.
Eye Irritation Testing: Draize Eye irritating test
Although cosmetic products aren’t directly put in the eye, the potential for accidental contact with products such as make up, shampoo and skin creams is high. Just like the skin, the tissue of the eye can become inflamed when exposed to irritants, sensitizers or photosensitizers
Test animal is Rabbit, as it does not shed tear, so a test sample placed in the eye is not diluted.
Procedure: Test sample is injected in one eye, other eye serves as a control. The sample is left for 3 days. The eye of the rabbit is regularly inspected for any kind of irritation, reddening.
Inhalation Testing
When inhaled into the body, some compounds may cause internal damage or inhibit the respiratory system. Symptoms may include coughing, sneezing or burning. For this reason, any compound that might be used in an aerosol or particulate product should undergo inhalation safety tests. In this test, animals are exposed to the compound via inhalation at a certain conc. in the air. Mortality rates are then measured. This data will help in quantifying the usage of the compound.
Acne genecity Certain cosmetic products can also cause acne. Researches have found that the external ear canal of an albino (New Zealand rabbit) is a good model for predicting a products acnegenecity. The test product is applied to the rabbit’s ear and observed for acne daily for 2 weeks. Then the product receives a qualitative rating for its acnegenic potential. Although the data obtained for acnegenecity from animal testing is helpful, it cannot replace human testing.
Systemic Effects Compounds used in cosmetic products must also be tested for systemic effects such as mutagenecity (the ability to damage DNA), carcinogenecity (ability to cause cancer) and teratogenecity (the ability to cause birth defects).These tests are long term and it continues for 2-3 yrs. The subjects are kept under observation for long term. One assumption about these tests is that, if an agent causes cancer, mutations or birth defects in animals, it is likely to cause the same problem in human beings.
INDIAN STANDARD: Note that in India, Animal testing is stopped thus one must follow IS 4011-Safety Evaluation Of Cosmetics. Depending on the product, prior human participant studies may be carried out in order to confirm the safety of the cosmetics finished product and to evaluate its tolerance in humans. Any human testing must be scientifically justified, as unjustified testing is unethical. The test should be conducted on selected panel of human volunteers, using good clinical practices, under the supervision of Dermatologist, and/or Ophthalmologist and/or Paediatrician (depending on the nature of the evaluation).
Performance /Efficacy testing
Efficacy tests are essentially designed to show that a product performs the function for which it is intended, for example that a moisturizer ‘moisturizes’, a deodorant reduces or masks detectable body malodour, an antidandruff shampoo reduces visible dandruff scale, and so on. In order to establish a frame of reference for the evaluation of efficacy it is usual to include both positive and negative controls in a study. The positive control is often a leading marketed product with an established history of effective performance. The negative control, in the simplest case, would be the test formulation minus the active ingredient, a true placebo.
Alternatively, the efficacy test may be simple comparison of new versus old, such as the comparison of novel and conventional moisturizing agents in a standard moisture lotion base.
Whenever possible the efficacy tests should be run on a double-blind basis to avoid the risk of bias in the evaluation procedure. This involves providing test and control products in identical plain containers distinguished by code numbers so that test subjects and accessories are not aware which sample is which. The code is revealed on completion of the study. This has the advantage of ensuring a totally independent and unbiased result.
In addition to product efficacy, the feedback on perfume, colour and Packaging is also important as it is rightly said “The best quality product in the world is not good if it does not sell, and it will not sell if it does not look right, or if the pack does not appeal to the customers”.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









