ABOUT AUTHORS:
Raj Kishor
Clinical Research Coordinator(CRC)
Tech Observer India Pvt Ltd
The Global CRO, N.Delhi
raryan859@gmail.com
ABSTRACT:
The Clinical trial design is the formulation of trials and experiments in Medical and epidemiological research. Clinical trials are research studies that involve patient or healthy people. They are designed to test new treatments such as drugs, vaccines, new approaches to preventing disease, surgery, radiotherapy, physical and psychological therapies and methods of diagnosing disease. The choice of trial design is always a critical decision in designing a clinical trial: That choice affects the inferences that can be drawn from the trial, the ethical acceptability of the trial, the degree to which bias in conducting and analyzing the study can be minimized, the type of subjects that can be recruited and the pace of recruitment, the kind of endpoint that can be studied, the acceptability of the results by the regulatory authorities, and many other features of the study, it conduct and its interpretation.
REFERENCE ID: PHARMATUTOR-ART-2069
INTRODUCTION:
Clinical trials are medical research studies to test whether different treatments are safe and how well they work[1,2]. Some trial are involves healthy members of the public and others involve patients who may be offered the option of taking part in a trial during the care and treatment—as in the cases of Cancer /HIV Patients[1].
Clinical trials aim to find the best way to:
* Prevent disease and reduce the number of people who become ill.
* Treat illness to improve survival rate or increase the number of people cured.
* Improve the quality of life of people living with illness; including reducing symptoms or the side effects of treatments, such as chemotherapy.
* Diagnose disease and health problem.
Clinical trials covers a wide range of different types of research – for example, Trials are often used to treat new medicines or vaccines but they can also be used to look at new combinations of existing medicines[1]. They can also be used to test whether giving a different treatment in a different way will make it more effective or reduce any side effects. Some trials are designed to try out ways to prevent a particular disease in a people who have never had the disease or to prevent a disease from returning.
During the latter stages of the treatment being developed, researchers will reports on the benefits and risks so that doctors can decide whether to use it, or how best to use it. It is important that results of the clinical trials are published so that people can use the informal to help them make decision about treatment and health care[2,3].
HISTORY:
Ancient
The Canon of medicine in 1025, he laid down rules for the experiments use and testing of drugs and wrote a precise guide for practical experimentation in the process of discovering and proving the effectiveness of medical drugs and substances. One of the famous trial was James Lind’s demonstration in 1747 that citrus fruits cures scurvy. He compared the effects of various acidic substances ranging from vinegar to cider, on groups of afflicted sailors and found the group who were given orange and lemons had largely recovered from survey after six days. Edward Jenner in the yr 1796 proved inoculation could prevent small pox, Jenner trial was the first step towards mass vaccinations that concurred epidemics such as typhoid, yellow fever, polio, measles. Frederick Akbar Mohamed in the yr 1884 made substantial contributions to the process of Clinical trials during his detailed clinical studies[4,5].
Modern
Evolution of Clinical Research:
* Syphilis Study--- 1932-72 and Belmont Report--- 1979
* Sulphonamide Tragedy--- 1937 and FD & Cosmetic act—1938
* Nazi experiment –1940-45 and Nuremberg code--- 1947
* Thalidomide tragedy---1953 and Kefauver-Harris amendment---1962
* Declaration of Helsinki—1964 and Statement of human experimentation---1966
* Therapeutic good act ---1989
* ICH-GCP---1997
* National statements---1999
* National statement code of research---2007
TRIAL DESIGN:
Clinical trials are the best way to compare different approaches to preventing and treating illness and health problems. Health professionals and Patients need the evidence from trials to know which treatments work best. Without trials there is a risk that people could be given treatments which have no advantage, waste resources, and could be harmful. Many treatments that are now commonly used were tested in clinical trial[6].
Some types of Clinical trials are designed to look at a treatment at an early stage of development. Researchers and Regulators will look at the information gathered from the trial and decide whether it is safe and appropriate to continue the developing the treatment. If, the treatment has no benefit, or has serious side effects, it may not be developed further[2].
How to design and analyze?
One needs to ask several questions, for examples:
* What are the objectives and end point of the study?
* What patient population or disease is; the drug mean to treat?
* What criteria should be used to select patient eligibility for the study?
* How large should the sample size be so that study will have enough power to detect a clinically significant benefit?
* How sure can we be about the observed treatment benefits and that will reflects a genuine treatment difference with minimal influence of systematic errors, confounding?
Who runs the Clinical trials? Clinical trials run under the supervision of doctors, Researcher and Specialists.
Participating Number of centers:
Clinical trials can also be classified as single-center or multi-center studies according to the no. of sites involved. While single-center studies are mainly used for Phase-I,II studies, Multi-centric studies can be carried out at any stage of clinical development.
Multi-centric studies are necessary for two reasons:
* To evaluate a new medication or procedure more efficiently in terms of accruing sufficient subjects over a shorter period of time.
* To provide a better basis for the sufficient generalization of the trial’s findings i.e effects of the treatment are evaluated in many types of centers.
TRIAL DESIGN SETUP: Doctors, Nurses, and Researchers work together with statisticians, trial manager and representatives from Pharmaceutical companies, if relevant, to design the best possible trial[5,6].
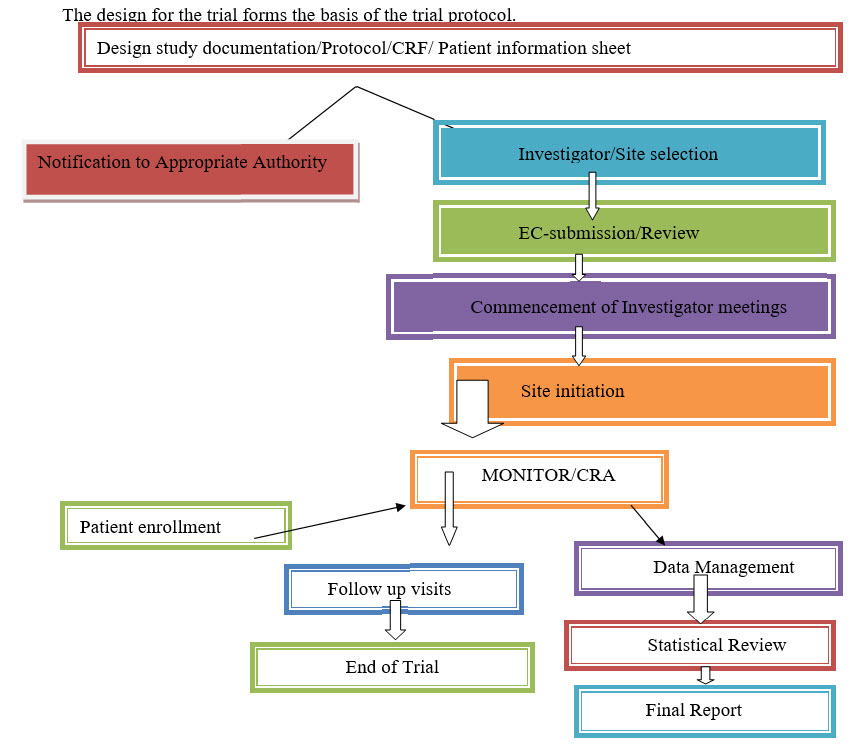
Who is going to be recruited in the trials: Eligibility- Inclusion and Exclusion Criteria?
Typical inclusion Criteria:
* Subject/Participants must have disease of Interest.
* Subject must have a certain amount of disease.
* Subject must understand study and agree to participate.
Typical Exclusion Criteria:
* Subject must not be on active treatment.
* Subject must not be allergic to intervention.
* Pregnancy, Breastfeeding, Child.
Study procedure:
* Divide your study patients in to groups.
* Administer the study and control interventions to the respective groups.
* Measure the Outcome.
TYPES OF SCIENTIFIC TRIALS:
[1]. Descriptive trials: Hypothesis generation.
[2]. Analytical or Observational trial:Relate associations between causes and diseases.
[3]. Experimental trials: Demonstrate efficacy and Safety.
Descriptive trials: Useful in generating hypothesis, simple and inexpensive. Describe and document Characteristics of Human disease, Personal experience, unexplained phenomena, and innovative treatments[2,6,7].
Hypothesis: The hypothesis is a clear statement of what is intended to be investigated. It should be specified before research is conducted and openly started in reporting the results. This allows identifying the research objectives / its relation to both the problem statement and literature review.
Types of hypothesis
[1] Null hypothesis—Designated by HO or HN
[2] Alternative hypothesis-- Designated by H1
Null hypothesis:Represents a theory that has been put forward, either because it is be-lived to be true or because it is to be used as a basis for argument, but hasn’t been proved.
Alternative hypothesis: The alternative hypothesis is a statement of what a hypothesis is test is set up to establish. Opposite of Null. Only reached if HO is rejected.
Let’s see – In a Clinical trial of a new drug, the null hypothesis might be that the new dug is no better, on average, than the current drug.
We would write HO: There is no difference between the two drugs on average.
We would write H1: The new drug has a different effect, on average, compared to that of the current drug.
Case series studies: It is an uncontrolled retrospective review of a group of patients. It is a cross sectional in nature as treatment and outcome are determined simultaneously at a single point of time. These studies are relatively in expensive. Results can be obtained quickly. It is most hypothesis generator.
Analytical Trial: Seek causes, etiologies, predictions, better diagnosis, assess therapy. The Investigator is observing nature rather than controlling treatment allocation[6,7].
Types:
* Case control study – Looking backward.
* Cross sectional Study– At present
* Cohort study – Looking forward
Case control study: Begins with cases (People with the disease). Go retrospectively from the disease to the risk factor. Select controls from the same population. Further data are then collected on those individuals and then groups are compared to find out if other characteristics are also different between groups. Determine the presence or absence of risk factors in two populations. Association determined by the odd ratio ([>1).
Cross sectional Study: Begins with a study population. Classify the population in terms of the presence or absence of the outcome, determining the prevalence of the abnormality (e.g community survey of asymptomatic bacteria). Determine what risk factors are present in two groups[13,14]
Advantages—Efficacy, with all information collected at the same time often from immediately available sources.
Prevalence: Measures how much of some disease or condition there is in a population at a particular point of time. Prevalence is calculated by dividing the number of persons with the disease or condition at a particular time by the number of individuals examined.
Incidence: Measures the rate of occurrence of new cases of disease or condition[11,12]. Incidence is calculated as the number of new cases of a disease or conditions in a specified time period(Usually a yr) divided by the size of population under consideration who are initially disease free.
Cohort study: A cohort study is a group of people who share a common characteristics or experience within a defined period (e.g are born, are exposed to a drug/vaccine). Go prospectively from risk factors to the outcome. Measures of outcome often requires years of follow up. More expensive than case control studies. Association determined by the relative risk (>1).
Experimental trials /Randomized Clinical Trials (RCT): Randomization assurance that subject populations are similar in test and control groups is best attained by randomly dividing a single sample population in to groups that receive the test or control treatments. Randomization avoids systematic difference between groups with respect to know or unknown base line variables that could affect outcome. Participants allocated randomly to either receive a specific intervention. Randomization and blinding are the two techniques usually used to minimize the chance of such bias and to ensure that the treatment and control groups are similar at the start of the study and are treated similarly in the course of the study.
Bias: The systematic tendency of any aspects of the design, conduct, analysis and interpretation of the results of the clinical trials to make the estimate of a treatment effect deviates from its true value.
Compare outcomes (etiology, Cause, efficacy) or trial group and control group following an intervention. Most powerful tool assess efficacy. Controlled, randomized, double-blind trails are the ‘’GOLD STANDARD’’ in clinical research.
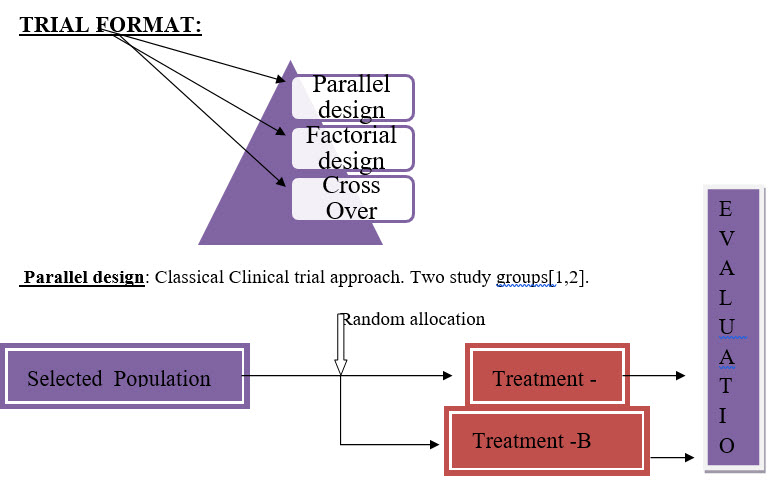
Classification:
|
S.No |
Based on |
Classification |
|
1. |
Type of intervention |
Therapeutics; Preventive |
|
2. |
Unit of Randomization |
Individual ; community |
|
3. |
Design |
Parallel; Cross Over; Factorial |
|
4. |
Sample size |
Fixed; Sequential |
|
5. |
Randomization |
Fixed; Adaptive |
|
6. |
Masking |
Single; Double; Triple blinding |

There are three basic ways to generate a randomization scheme for an RCT.
1. Simple randomization
2. Block randomization
3. Stratified blocks
Simple randomization: The simple randomization, which is equivalent to tossing a coin for each subject that, enters a trial. The heads get the experimental treatment while the tails receive the placebo. A computerized or tabulated random number generator is generally used. It is simple and easy to implement and treatment assignment is completely unpredictable[15].
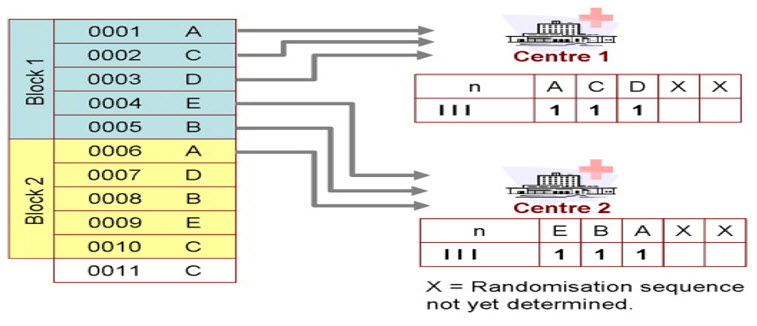
Block randomization: It is very popular and balanced within each block. For a trial of n treatments, the total number patients are divided into m blocks of size 2n. Then, each of the m blocks is randomized such that n patients are allocated to each of the treatments. One can then choose the blocks randomly. The INVEST study followed this scheme of block randomization[16].
Stratified blocks: A third approach to randomization involves “stratified blocks.” Because a trial may not be considered valid if it is not well balanced across the prognostic factors, stratification of patients is done to produce comparable groups with regard to certain characteristics (e.g., gender, age, race, disease severity). This approach produces valid statistical tests in all stratified subgroups (e.g., high-risk subgroups in the ALLHAT trial)[17,18]
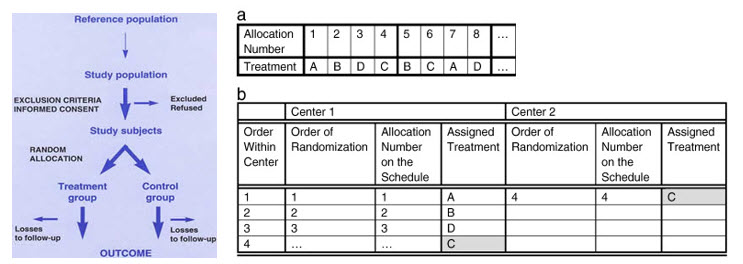
Whatever the mode of randomization is, it is ensured that the pattern of assignment of control or experimental drug within a group of patients cannot be guessed at any point. It is recommended that the statistician who generated the randomization codes does not get involved in the IA or the final analysis of the experimental data[18,19].
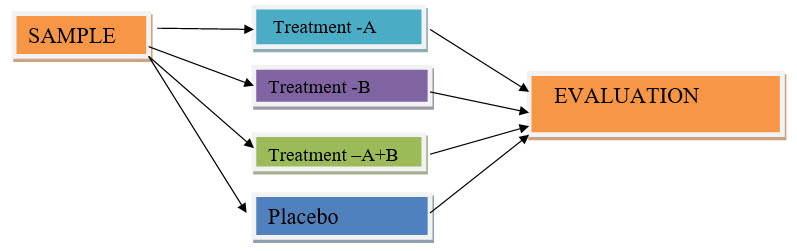
Factorial design: A simple factorial design would have one group testing therapy (A), another testing therapy (B), A third group testing (A) and (B) combined and a control group testing neither (A) nor (B). Factorial designs are considered an efficient way to test medicines in combination, but their results are not always easy to interpret[1,2].
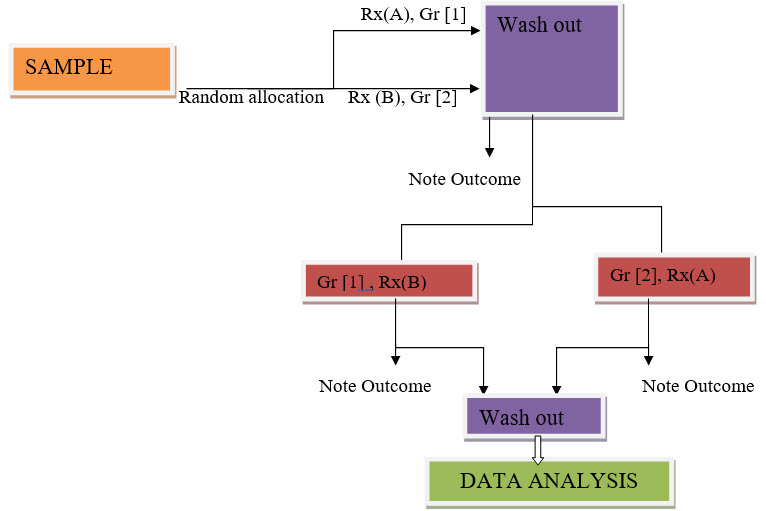
Cross Over: In a cross over trial, each participant get both treatment being treated. Some participants are assigned at random to receive drug (A) and later drug (B). Other receive (B) then (A). To produce valid results, the effect of the first drug must end before the 2nd drug taken, and vice versa. This recruitment can hard to satisfy andis one reason cross over trials are not used[2].
DIFFERENT PHASES OF TRIALS:
* Phase – I
* Phase- II
* Phase- III
* Phase- IV
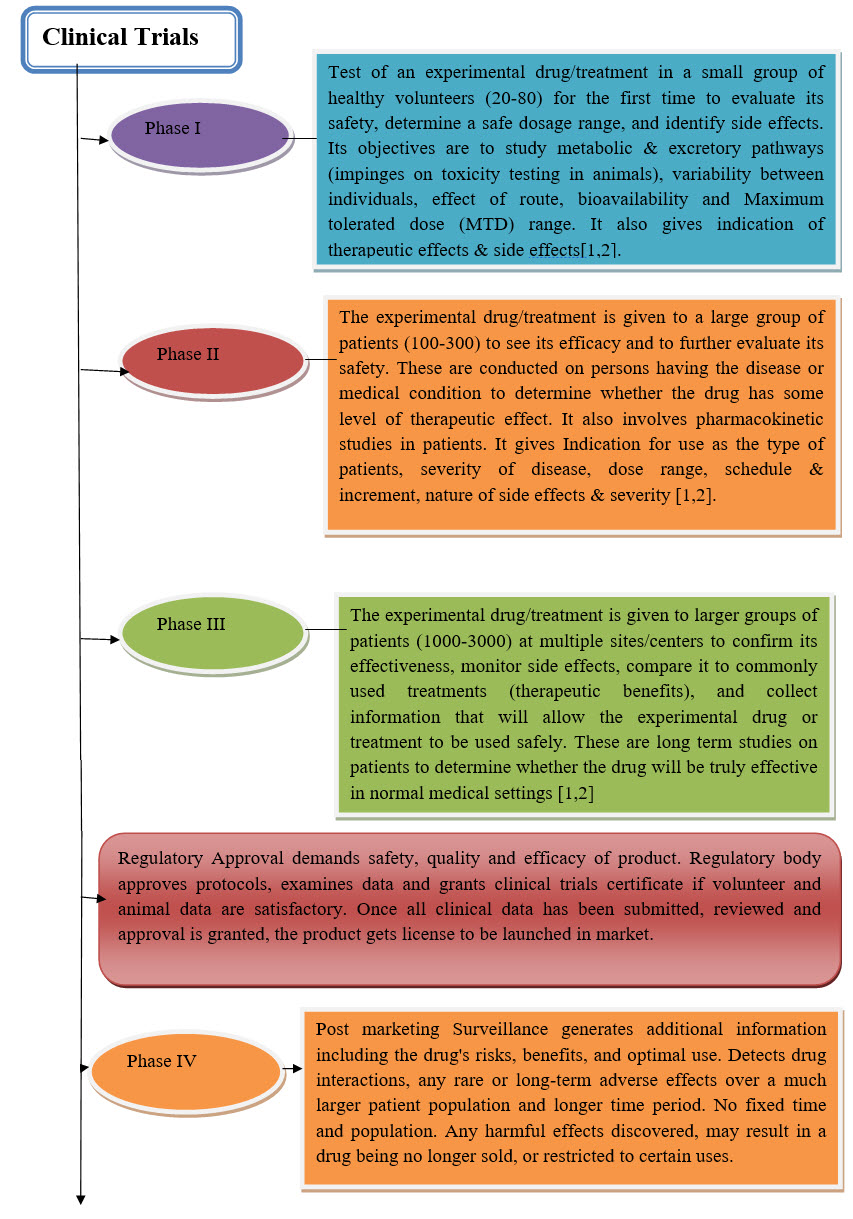
Phase – I: Early use of drug. Mainly aimed in establishing safety and toxicity limits. Study subjects often normal. No control groups. Usually open level[20].
Phase I design: Based on tradition, not so much on statistical theory. Dose escalation is reach maximum tolerated dose (MTD). Dose escalation often based on Fibonacci series i.e 1 2 3 5 8 13….
Let’s see:
[1] Enter 3(5) Patients at a given dose.
[2] If no toxicity, go to next dosage and repeat step 1.
[3] a. If 1 patient has serious toxicity, and 3 more patients at that dose (go to 4)
b. If 2/3 have serious toxicity , consider MTD.
[4] a. If 2 or more of 6 patients have toxicity, MTD reached.
b. If 1 of 6 has toxicity, increase dose and go back to step1.
Phase- II: Proof of principal stge. Intervention studied in patients with diseases. Dose findings. More safety data. Sometimes combined with Phase- I.
Let’s see: LetX% = 20% i.e want to check if drug likely to work in at least 20% of patient.
[1] Enter 14 Patients
[2] If 0/14 responses – No responses are observed trial is terminated.
[3] 1+/14 responses. Add 15-40more patients.
[4] Estimate response rate.
Phase- III: Randomized controlled trial. Double blind whenever possible. More patients. Multi center. Ongoing safety data.
Phase III design: Comparative study : Experimental Vs Control group
Phase- IV: Post approval studies. Further exploration of Intervention - -New Indications or new uses of drugs.
DATA ANAYLSIS:
Record the outcome(s) of Interest. Compare the data for each intervention group. Derive conclusions.
CLINICAL TRIAL REPORTING:
A written description of a trail/Study of any therapeutic, prophylactics or diagnostic agent conducted in human subjects, in which the clinical and statistical description presentation and analyses are fully integrated in to single report[6].
CONCLUSION:
Research and the Clinical trial is a fundamental of modern medicine. In fact, the Clinical trial is the medical invention that has contributed to nearly all of the life saving medicine.
These medicines have: Added 10yrs to our life expectancy, Made organ transplant possible/ Diabetes manageable conditions. Extended the life expectancy of AIDS patients and prolonged the lives of millions of Cancer patients.
REFERENCES:
[1] Duolao Wang, Ameet Bakhai, ‘’Practical guide to Design, Analysis and Reporting’’, Published by Remedica common wealth house,UK: 2-3.
[2] Professor Janet Darbyshire, Sir Iain Chalmers, Dr Sophie Petit-Zeman, Roger Wilson, Dr Marianne Miles, Dr Matthew Hallsworth ‘’Understanding Clinical Trial- Booklet Version-2 (October2010)’’ .
[3] crncc.nihr.ac.uk/Resources/NIHR%20CRN%20CC/Documents/Brochures/Understanding%20Clinical%20Trials%20Booklet%20-%20crystal%20mark.pdf
[4] en.wikipedia.org/wiki/Clinical_trial
[5] clinicaltrials.gov/ct2/about-site/history
[6] fda.gov/downloads/Drugs/NewsEvents/UCM251958.pdf
[7] cel.sfsu.edu/clinical-trials/
[8] Information from the NHS about clinical trials
ibrary.nhs.uk/trials
[9] MRC Clinical Trials Unit
ctu.mrc.ac.uk/about_clinical_trials.aspx
[10] Database of ongoing and completed clinical trials in the UK
controlled-trials.com/mrct
[11] UK Clinical Trials Gateway
controlled-trials.com/ukctr
[12] Directory of clinical trials funded by the pharmaceutical industry
ifpma.org/clinicaltrials
[13] The U.S. National Institutes of Health ClinicalTrials.gov
clinicaltrials.gov
[14] Opportunities for public involvement in clinical research
peopleinresearch.org
[15] Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy.(RENAAL) N Engl J Med. 2001;345:861–9.
[16] Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): A multicentre randomised controlled trial. Lancet.2005;366:895–906.
[17]. Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, Wasserman K, Oudiz R, Shapiro S, Robbins IM, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2003;41:2119–2125.
[18] Center for Drug Evaluation and Research. Medical review of application #22–081: Ambrisentan. Center for Drug Evaluation and Research; 2007.
[19] McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, Badesch DB, Barst RJ, Hsu HH, Rubin LJ. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006;174:1257–1263.
[20] Sue-Jane Wang, Clinical Trial design/ ‘’CDER small Business Assistance Clinical Trial forum’’April 21, 2011.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









