ABOUT AUTHORS:
Bhavesh B. Patel*1, Nilam A. Patel2
1Department of Industrial Pharmacy
2Department of Pharmaceutics and Pharmaceutical technology
Shree S. K. Patel college of pharmaceutical education and research,
Ganpat University, Kherva, Mehsana – 384012, Gujarat.
*pbhavesh11@gmail.com
ABSTRACT
Nasal drug delivery has always been an area of research for industries because of its benefit in both local as well as systemic delivery of various therapeutic and debatably a drug delivery route to the brain. To use this route several parameters are being considered like the nature of disease condition (acute or chronic) and intended effects (local, systemic or central nervous system (CNS)) of drug treatment. Which formulation is better to target brain through nose would also be considered, device for effective nasal to brain drug delivery is also a develop thrust area for device companies. By nasal route BBB can be avoided, rapid action can be given in diseases like migraine, brain tumor, Alzheimer, Parkinson’s disease and hormone replacement. Direct delivery of intranasal drugs to the brain has been proposed, but is not universally established.
REFERENCE ID: PHARMATUTOR-ART-1816
INTRODUCTION
Many nasally applied dosage forms are available in the market. Two third are locally acting decongestants, antihistamines or cortisones, and one third comprises a number of antimigrane drugs, peptide hormone analogs and nicotine nasal spray, all with systemic effects. Diseases of the Central Nervous System (CNS) that require drug treatments are numerous; schizophrenia, meningitis, migraine, obesity, Parkinson’s disease and Alzheimer’s disease and are all such examples. Drug delivery to the brain, however, remains problematic because of poor bioavailability from the blood due to the impervious nature of the endothelial membrane separating the systemic circulation and central interstitial fluid, termed the Blood Brain Barrier (BBB).[1] This is a major impeding factor to progress in the field. Hence, many potent therapeutic agents may have been abandoned because sufficient drug levels in the brain can not be achieved via the blood. A well described strategy to promote BBB penetration is to derivatise small molecular weight drugs into pro-drug analogues which are more lipophilic and so allow passive diffusion of the pro-drugs across the BBB. These are later converted by enzymes to the native form. [2] In recent years it has been shown in the literature from animal and human investigations that transport of exogenous materials directly from nose-to-brain is a potential route for by-passing the BBB. [3] This route, termed direct nasal cavity to brain drug delivery, involves the olfactory or trigeminal nerve systems which initiate in the brain and terminate at the olfactory neuroepithelium or respiratory epithelium (respectively) in the nasal cavity. If drugs could reach the CNS by this route then it would not only regenerate interest in previously abandoned drug compounds but also enable an entirely novel approach to CNS drug delivery. Recently, several nasal formulations, such as ergotamine (Novartis), sumatriptan (GlaxoSmithKline), and zolmitriptan (AstraZeneca) have been marketed to treat migraine. Scientists have also focused their research toward intranasal administration for drug delivery to the brain especially for the treatment of diseases, such as epilepsy[15,20-24] [4-9], migraine[13,14,18,25-27] [10-15], emesis, depression[28] [16], angina pectoris[29] [17] and erectile dysfunction[30] [18].. This system is a part of nasal drug delivery system which have-
ADVANTAGES
- Non-invasive, rapid and comfortable
- Bypasses the BBB and targets the CNS, reducing systemic exposure and thus systemic side effects
- Rich vasculature and highly permeable structure of the nasal mucosa greatly enhance drug absorption
- Does not require any modification of the therapeutic agent being delivered
- Works for a wide range of drugs. It facilitates the treatment of many neurologic and psychiatric disorders
- Problem of degradation of peptide drugs is minimized up to a certain extent
- Easy accessibility to blood capillaries
- Avoids destruction in the gastrointestinal tract, hepatic “first pass” elimination and gut wall metabolism, allowing increased, reliable bioavailability.
LIMITATIONS
- Concentration achievable in different regions of the brain and spinal cord, varies with each agent
- Delivery is expected to decrease with increasing molecular weight of drug Some therapeutic agents may be susceptible to partial degradation in the nasal mucosa or may cause irritation to the mucosa
- Nasal congestion due to cold or allergies may interfere with this method of delivery
- Frequent use of this route results in mucosal damage (e.g. infection, anosmia).
HOW CAN BE BRAIN TARGETED BY NASAL ROUTE
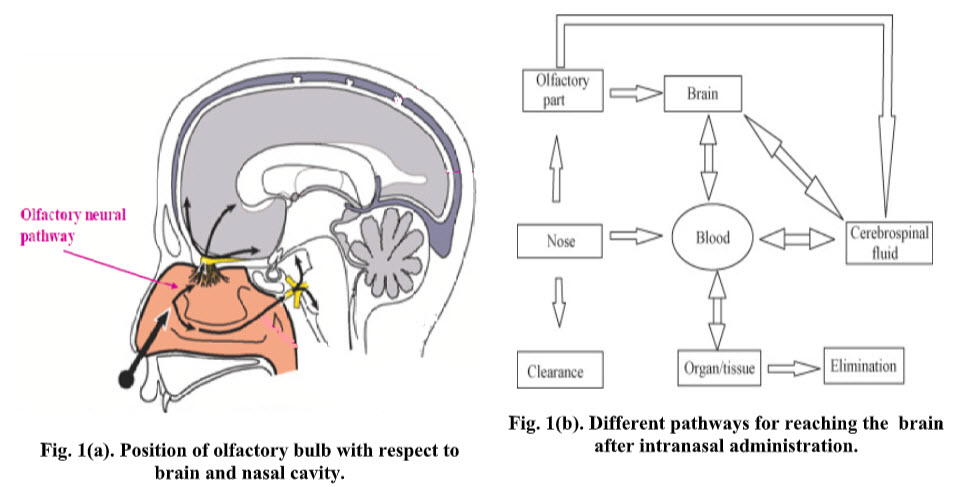
In the recent years several drugs as well as peptides have been delivered effectively using intranasal route. Administration of NAD+ greatly decreased brain injury in a rat model of transient focal ischemia and profoundly decreased oxidative cell death. [24] Similarly intranasal administration of gallotannin, a poly (ADP-ribose) glycohydrolase (PARG) inhibitor showed a marked reduction in the frequency of ischemic brain injury in rats. [25] Olanzapine when delivered intranasally as mucoadhesive microemulsion formulation showed better effectiveness of the route of drug delivery into brain.[26][27] The delivery of buspirone hydrochloride as mucoadhesive formulation using chitosan and hydroxylpropyl beta cyclodextrin showed better brain concentration after intranasal administration in mice. [28] Similarly intranasal mucoadhesive microemulsion of sumatriptan showed better cerebral concentration and reduction in migraine headache. [29]
DIRECT BRAIN DELIVERY
Various newer approaches have been presented for the direct delivery of drug molecules to the CNS. These approaches have the advantage of delivering much higher concentration of neurotherapeutics by directly injecting the drug into CSF or parenchymal space subsequently reducing the drug concentration in the peripheral environment. [30] Several factors that monitors the CSF concentration includes drug volume distribution, site of puncture, rate of clearance, drug diffusion, transport pathways and CSF production rate.[31] Several approaches that have been developed for direct delivery of drug molecules and peptides into CNS include intracerebral, intraventricular, transcranial, intrathecal, intraparenchymal (intra CSF) delivery, polymer depot formation and BBB disruption.
To understand the mechanism, pathways, distribution and absorption of therapeutic agents administered to the CNS by the intranasal route, a brief description of the nasal physiology is considered necessary.
NASAL PHYSIOLOGY
The nose is divided into two nasal cavities via the septum. The volume of each cavity is approximately 7.5 mL and has a surface area around 75 cm. [32-34] There are three different functional regions in the nose-vestibular, respiratory, and olfactory. Of these, the respiratory region is the most important for systemic drug delivery.[34]The respiratory epithelium consists of basal, mucus-containing goblet, ciliated columnar and non-columnar cell types. [34-35] The Celia move in a wavelike fashion to transport particles from the pharynx area for ingestion.[34] [36] Additionally, the cells in this region are covered by 300 microvilli, providing a large surface area for absorption. [34]Below the epithelium is lamina propria. This is the region where blood vessels, nerves, serous glands, and mucus secretory glands may be found.[35]The lamina propria also houses a dense network of capillaries, many of which are very permeable for drug absorption. [33] [37]
The nasal epithelium is covered by a mucus layer that is renewed every 10 to 15 min.[37] The pH of mucosal secretion ranges from 5.5 to 6.5 in adults and from 5.0 to 6.5 in children. [38] The mucus layer entraps particles, which are then cleared from the nasal cavity by the cilia. The rate of mucus flow through the nose is approximately 5 to 6 mm/min resulting in particle clearance within the nose every 20 min.[33] [39]
The nasal cavity also houses numerous enzymes.[39-41]In humans, cytochrome P450 enzyme isoforms that have been identified are CYP1A, CYP2A and CYP2E.[42]Other enzymes detected in the human nose include carboxylesterases and glutathione S-transferases.[43-45].
In addition to its function as a passageway for respiration, the nasal cavity also has a key role in the sense of smell. The olfactory nerves, which originate as specialized olfactory nerve endings (chemoreceptors) in the mucous membrane of the roof of the nasal cavity above the superior nasal conchae, are the sensory nerves of smell. On each side of the septum nerve fibers pass through the cribriform plate of the ethmoid bone of the olfactory bulb where interconnections and synapses occur. From the bulb, a bunch of nerve fibers pass through the olfactory tract and reach the olfactory area in the temporal lobe of the cerebral cortex in each hemisphere, where the impulses are interpreted and odor is perceived. Another set of nerves emanating from the nasal cavity is the maxillary branch of the trigeminal nerves, which are general sensory nerves [46]
MECHANISMS
Two mechanisms are involved in the nasal delivery, a fast rate that depends on lipophilicity, and a slower rate that depends on molecular weight. McMartin et al studied [47] the transport of SS-6 (an octapeptide) and horseradish peroxidase through a rat's nasal cavity. Their absorption studies are consistent with the non-specific diffusion of the penetrant molecules through aqueous channels located between the nasal mucosal cells, which impose a size restriction on nasal permeability. The data indicate that good bioavailabilities can be achieved for molecules up to 1000 dalton (Da) (without enhancers) and good availability can be extended to at least 6000 Da with enhancers. The transport mechanisms of different substances like insulin, mannitol or propranolol across the nasal mucosal tissue were studied by Wheatly et al.[48]The transport of these substances occurs by a passive transport mechanism. The addition of deoxycholate (0.1%) reversibly increased the transepithelia conductance across the nasal membrane and enhanced the transport of mannitol and insulin. The transport of tyrosine and phenylalanine across rat mucosa was also studied by using an in-situ perfusion technique. [49] It was found that both amino acids were absorbed by an active saturable transport process, which appeared to be Na+ dependent, and transport may have required metabolic energy as a driving force. Water-soluble substances such as sodium cromoglycate are absorbed well and nasal absorption probably depends on aqueous channel diffusion (pores). [50] The molecular size of the compound will be a determinant in the rate of absorption in such a channel.
PATHWAYS
Two broad mechanisms of drug transport through the epithelial cell layer have been identified, namely, paracellular (between epithelial cells) or transcellular (apical to basolateral transport through epithelial cell).
The paracellular route comprises of a hydrophilic channel that allows only the smallest of drug molecules to pass through it due to the restrictive nature of the junctional complexes that link the epithelial cells. In nasal epithelia the largest molecular size for paracellular drug transport has been estimated as 1000dalton(Da). [51] Hence, dopamine[52] [53] and morphin [54] are both believed to pass paracellular (as well as trancellularly) between olfactory epithelial cells. However, tight junctions are generally impermeable to molecules with a hydrodynamic radius greater than 4-8Å (depending on the ‘leakiness’ of the epithelium) which restricts the movement of molecules larger than this to pass between neighbouring cells. [51] Hence, nanoparticles are too large for this route to be of use. Therefore, since the main focus of this review is to evaluate the particulate delivery of drugs directly from nose-to-brain, the rest of this section will concentrate on the transcellular route. The transcellular route is less well characterised than the paracellular route. [55] However, novel spectroscopy and microscopy techniques such as electron energy loss spectroscopy and energy filtering transmission electron microscopy are providing new insights into this complex process. [56] Lipophilic, small molecular weight drugs are able to passively transport through the cell membrane. The aim of this section, however, is to describe the different endocytic routes of transcellular uptake of drugs and nanoparticles by cells, and speculate how they may influence direct N-B drug delivery. Endocytosis has been categorised by a number of different molecular mechanisms including macropinocytosis, clathrin-mediated, clathrin-independent, caveolinmediated, caveolin-independent and phagocytosis [57] (Figure 2). Macropinocytosis is an endocytic mechanism where the action of actin filaments gives rise to curved ‘ruffles’ on the cell surface. Sealing of the aperture into discrete vacuoles forms the macropinosome (0.5-5μm diameter) which efficiently takes up extracellular fluid into the cell. [58] Considerable volumes of dissolved molecules and particles can be taken up in this way. Macropinocytosis is generally thought of as a constitutive process by which the cell can sample the extracellular environment and is not believed to be initiated by receptor activation at the cell surface. [58]
Receptor-meditated endocytosis is a term used to describe a group of endocytic mechanisms where the ‘cargo’ is thought to stimulate the endocytic event by complementing a receptor on the cell membrane. Receptor-mediated endocytosis can involve either clathrin-dependent or independent mechanisms. The clathrin protein is endogenously expressed within mammalian cells in the form of a heavy chain and a light chain. The fusion of these chains results in the formation of a triskelion structure, a 3D array of which produces a clathrin coat. [59] Recruitment of the coat to the cytosolic cell membrane gives rise to regions called ‘coated pits’ during the initial
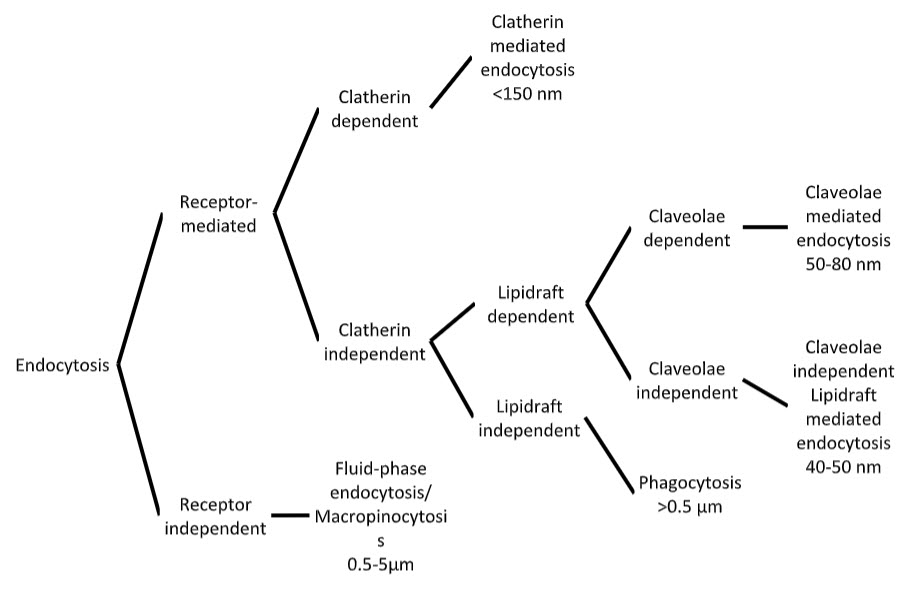
Figure 2. Cellular endocytic pathways (adapted from (Xiang et al 2006))
stages of receptor-mediated endocytosis. Invagination of the pit results in a clathrincoated vesicle that is taken into the cytosol. These vesicles can take various shapes and sizes but are generally believed to be <150nm. [58] Clathrin-dependent endocytosis is currently the best characterised endocytic mechanism; however, there is still debate over exactly how the cargo initiates the event. [60] Clathrin-independent endocytosis may involve cholesterol-enriched microdomains in cell membranes called ‘lipid-rafts’. Caveolae-mediated endocytosis is dependent on lipid rafts for its function. Caveolae are flask-shaped structures (50-80nm) that are rich in proteins and lipids such as cholesterol and sphingolipids and may be involved in signal transduction. [61] Caveolae-independent lipid raft-dependent endocytosis also exists and has recently been identified to involve microdomains (40-50nm) that contain a protein called flotillin.[62] But this mechanism is not yet fully understood.
A form of clathrin-independent endocytosis that does not involve the formation of lipid rafts is phagocytosis. This is a receptor-mediated uptake of exogenous materials by specialised phagocytic cells such as macrophage. This route of uptake leads to internalisation of relatively large (>1μm) patches of membrane [63] and engulfment of the exogenous material into lysosomes either for destruction or antigen presentation. Hence, phagocytic cells act to destroy materials that may be harmful to other cells and also alert immune system cells to their prescence. Phagocytic cells make up a minority of the total cell population in the nasal cavity and therefore are not thought to contribute to cellular uptake in therapeutic concentrations. [64] Furthermore, processing of drugs by the lytic enzymes and acidic pH conditions present in the lysosome may reduce drug potency. Consequently, other forms of endocytosis (macropinocytosis and clathrin-mediated endocytosis) represent more promising trancellular routes of drug uptake into cells that may allow therapeutic levels of drug to enter the body without substantial damage to the drug molecule.
Overall, in many cases, it is not yet fully understood how substrates such as nanoparticles initiate the process of cellular internalisation. Moreover, some commentators have even questioned the existence of a ‘cargo specific’ mechanism of uptake. [60] However, the lack of clear understanding of endocytic mechanisms should not dampen optimism. Knowledge from novel microscopic and spectroscopic techniques continually adds to the understanding of endocytosis. [65] Phagocytosis may lead to damage of the drug molecule, however, other lipid raft-dependent mechanisms (which include caveolae or flotillins) form vesicles that could deliver very small nanoparticles (40-80nm diameter) into the cells. Moreover, clathrin-mediated and macropinocytosis vesicles are larger (<150nm and 0.5-5μm, respectively) and allow 40-50nm passage of nanoparticles into cells in greater quantities which could be of therapeutic benefit. A better understanding of how these later two processes initiate could lead to the development of nanoparticles that can target these routes of uptake. With respect to nose-to-brain drug delivery of nanoparticles it is clear that the delivery of different nano-sized particles may lead to uptake of the drug carriers into different endocytic pathways. The fate of the nanoparticles is dependent on one or more of these individual systems. Hence, altering nanoparticle size and composition could be useful approaches to improving their cellular uptake into olfactory epithelial cells.
FORMULATIONS FOR INTRANASAL DELIVERY
Intranasal delivery devices; Droppers liquid dosage forms; Instillation catheters; Unit dos containers; Squeeze bottles; Pump spray; Compressed air nebulizer; Metered dose inhalers; Pressurized metered dose inhalers; Insufflators; Airless preservative free spray Solution: Suspension; Emulsion; Microemulsion; Nanoemulsion; (As nasal drops, nasal spray etc.)
Semisolid dosage forms: Gels; Ointments
Solid dosage forms:Microspheres; Nanoparticles; Powders
APPLICATION OF NASAL DRUG DELIVERY SYSTEM FOR BRAIN TARGETING
Delivery of protein therapeutic agents /macromolecules to CNS
In the age of advanced peptide, protein, and vaccine research, nasal administration of such compounds provides an attractive delivery route. In case of oral administration, the bioavailability of protein molecules tends to be relatively low due to their large molecular size and rapid enzymatic degradation.[66] Because of their physicochemical instability and susceptibility to hepato-gastrointestinal “first pass” elimination, peptide/protein drugs are generally administered parenterally. It is on this background that intranasal administration seems a promising option. Most nasal formulations of peptide/protein drugs have been made up in simple aqueous or saline solutions with preservatives. Recently, more R&D work has been directed towards the development of nasal drug delivery systems for peptide/proteins. Currently, in the United States only four intranasal pharmaceutical products for systemic delivery have been marketed i.e. desmopressin, lypressin (Diapid), oxytocin (Syntocinon), and nafarelin acetate (Synarel).
Delivery of protein therapeutic agents to the CNS clearly involves extraneuronal transport as it occurs within minutes rather than hours. A number of protein therapeutic agents have been successfully delivered to the CNS using intranasal delivery in a variety of species. Neurotrophic factors such as NGF,[67-69]IGF-I, [70]FGF [71] and ADNF12 have been intranasally delivered to the CNS in rodents. [72] Studies in humans, with proteins such as AVP, [73] CCK analog, [74] MSH/ACTH [75-76] and insulin [77-78] have revealed that they are delivered directly to the brain from the nasal cavity. Hussain [79] recently reviewed animal models to study nasal absorption and the effect of physico-chemical and biopharmaceutical properties of drugs on the rate and extent of absorption. The review also discusses factors affecting peptide absorption and methods to improve the nasal bioavailability of peptides. The bioavailability of protein molecules tends to be relatively low due primarily to their large molecular size and rapid enzyme degradation. As the number of amino acids increases beyond about 20, bioavailability becomes very low. [66]To overcome these issues, much research has been conducted in the areas of absorption enhancers and bioadhesive agents. Absorption enhancers are used to increase the bioavailability. They are basically surfactants, glycosides, cyclodextrin and glycols. They improve absorption through many different mechanisms, such as increasing membrane fluidity, increasing nasal bloodflow, decreasing mucus viscosity, and enzyme inhibition. [80]The classic example of a polypeptide compound with low nasal bioavailability is calcitonin. Its molecular weight is approximately 3,500 daltons and contains 32 amino acids in length. When calcitonin was given intranasally to rats and rabbits using a number of different cyclodextrins, its absorption as measured by a decrease in serum calcium concentration, was significant in comparison to the formulation without additive. [81] Another technique aimed to increase nasal absorption is the utilization of bioadhesive agents. These compounds promote binding of drugs to biological material in the nasal cavity, thereby extending their residence time and allowing increased absorption. Common bioadhesive materials are carbopol, cellulose agents, starch, dextran, and chitosan. [82-87] Liu et al [88-89] have demonstrated the therapeutic benefit of intranasal delivery of proteins in stroke studies. They have shown that intranasal IGF-I reduces infarct volume and improves neurologic function in rats with middle cerebral artery occlusion (MCAO). A preliminary report indicates that intranasal treatment is effective even when delayed for 4 h after the onset of MCAO. [90] Gozes et al [91] have shown that intranasal administration of a Vasoactive Intestinal Peptide Analog (VIP analog, containing 28 amino acids) prevented learning and memory impairments resulting from cholinergic blockade in rats treated with aziridium. They also demonstrated that a nine amino-acid fragment of ADNF (ADNF-9) and an ANDF-like peptide (NAP) also protected against short-term memory loss in the same animal model. Research in humans has also provided evidence of direct delivery of macromolecules to the CNS from the nasal cavity. Kern et al [92] have demonstrated CNS effects of intranasal corticotropin-releasing hormone (CRH) without altering plasma cortisol or CRH levels. Perras et al [93] have reported that intranasal delivery of growth hormone-releasing hormone (GHRH) not only increased rapid eye movement sleep and slow wave sleep in humans, but also decreased growth hormone. Al-Ghananeem et al [94] carried out a study on the utility of the nasal route for delivery of 17b-estradiol, using its water-soluble prodrug. The study revealed that CSF concentration of 17b-estradiol following intranasal delivery of prodrug was higher compared to an equivalent intravenous dose. This result has a significant value in the treatment of Alzheimer's disease. The efficacy of peptide/protein delivered intranasally is highly dependent on the molecular structure of the drugs and their size. Respiratory epithelial cells are capable of absorbing peptide/protein by a vesicular transport mechanism, followed by transfer to the extracellular spaces, and subsequent uptake by the submucosal vascular network. [95]
Delivery of DNA plasmids to the CNS
Of the several routes available for immunization, the nasal route is particularly attractive because of the ease of administration and the induction of potent immune responses, particularly in the respiratory tract. However, adjuvants and delivery systems are required to enhance immune responses following nasal immunization. The use of microparticles [poly(lactide co-glycolide)] as adjuvants and delivery systems for protein and DNA vaccines for nasal immunization were reviewed by Vajdy et al. [96] It has also been reported that after nasal administration of DNA plasmids, the level of plasmid in the brain was, 3.9 to 4.8 times higher than the plasmid concentration in the lungs and spleen. It was also found that the plasmid DNA reached the brain within 15 min following intranasal administration.[97] The higher distribution of plasmid to the brain after intranasal administration indicates that nasal administration might be a potential route for the delivery of therapeutic genes to the brain with reduced side-effects in the other organs. The plasmid administered in this study was very large as was the plasmid detected in the brain.
Delivery of small molecules to the CNS
Many small molecules have been shown to be transported directly to the brain and/or CSF from the nasal cavity. This has been reviewed by Illum[34]and Mathison et al.[36]Anand kumar et al[98] and David et al[99] have demonstrated intranasal delivery of estrogen and progesterone respectively, to the CSF. Studies have also shown that drugs such as L-NAME [100] and cocaine (at the lower end of the lipophilicity scale) [101] have a higher CSF and olfactory bulb concentration after nasal administration than that obtained after parenteral administration. The properties of small molecules, including size and lipophilicity affect delivery to the CNS following intranasal delivery. [102-104] Sakane et al [105] reported that following intranasal administration of the antibiotic cephalexin to rats, higher CSF concentration was reached at 15 min but it declined to approximately half that concentration at 30 min. Because cephalexin does not cross the BBB well and because CSF concentration was 166-fold higher after intranasal administration than after systemic administration in spite of similar blood levels, it was concluded that cephalexin entered the CSF directly from the nasal cavity. Using a series of fluorescein isothiocyanate-labeled dextrans (FITC-dextran) with increasing molecular weights, it was found that dextrans with molecular weights of up to 20,000 daltons could be transported directly from the nasal cavity of rats into the CSF.[100]The concentration of the FITC-dextrans in the CSF increased with decreasing molecular weight. These FITC-dextrans are not found in the CSF after intravenous administration. Similarly, a comparison of the brain olfactory bulb concentrations achieved 30 min after intranasal administration of 7.4 n mol dopamine (153 daltons) [106] with those obtained after intranasal administration of 7.4 n mol nerve growth factor (NGF) (26,500 daltons) [41-42]to rats, revealed a five-fold higher delivery of the lower molecular weight dopamine. Comparing the percentages of the original dose remaining in the brain 30 to 45 min after intranasal administration of dopamine (0.12%) [107] and NGF (0.023%) [69]in rodents revealed a similar difference. In addition, with most small molecules, a significantly higher molar dose can be delivered intranasally than with larger protein or DNA therapeutic agents. Thus, considerably higher concentrations of small molecules are achievable in the CNS with intranasal delivery. Ishikawa et al [108] reported that powder formulation of elcatonin utilizing CaCO3 improves the nasal bioavailability by increasing residence time in the nasal cavity and thus enhances the systemic bioavailability. Recently Bergstrom et al [109] studied the uptake of picolinic acid (PA) in the brain. [3H]PA was administered via unilateral nasal instillation or i.v. injection to mice. Autoradiography demonstrated rapid uptake of radioactivity in the olfactory nerve layer and in the ipsilateral olfactory bulb following nasal instillation, which was maintained at a high level even after 4 h. On the other hand i.v. injection of [3H]PA demonstrated selective uptake and retention of radioactivity in the olfactory bulb. Hussain et al[110] have found that intranasal administration of folic acid effectively results in complete and very rapid absorption into the CNS. This provides a method of rapidly and reliably delivering folic acid, alone or in combination with other compounds, to the systemic circulation to produce a beneficial effect in the treatment or prevention of Alzheimer's disease and stroke. Li et al[111]-reported rapid onset intranasal delivery of diazepam using ethyl-laurate-based microemulsion. At a 2 mg/kg dose, the maximum drug plasma concentration was arrived within 2-3 min, and the bio-availability (0-2 h) after nasal spray compared withi.v injection was about 50%. The results suggest that this approach may be helpful during emergency treatment of status epilepticus.
Illum et al [112] have studied the effect of chitosan-morphin nasal formulation vis-a-vis slow i.v. infusion of morphine in healthy volunteers who reported sedation at the earliest time point after nasal administration compared withi.v. administration. This suggests that after nasal administration morphine may be able to reach CNS more rapidly than afteri.v. administration.
CONCLUSION
Brain targeting is not easy due to BBB and sometimes enzymal degradation of therapeutic agents but nasal route may be effective to target brain directly or without through the systemic route. Olfactory region is the only part of brain which is located outside of the skull- in the nasal cavity. If olfactory lob can be targeted, it would be ease to deliver in to CNS.
REFERENCES
1.Pardridge, W. M. Non-invasive drug delivery to the human brain using endogenous blood-brain barrier transport systems. Pharmaceutical Science & Technology Today, 1999; 2: 49-59
2.Krishnamoorthy, R., Mitra, A. K. Prodrugs for nasal drug delivery. Advanced Drug Delivery Reviews, 1998; 29: 135-146
3.Illum, L. Transport of drugs from the nasal cavity to the central nervous system. European Journal Of Pharmaceutical Sciences, 2000; 11: 1-18
4.Vyas TK, Babbar AK, Sharma RK, Singh S, Misra, A. Intranasal mucoadhesive microemulsions of clonazepam: Preliminary studies on brain targeting. J Pharm Sci 2005; 95: 570-580.
5.Florence K, Agrawal HG and Misra A. Intranasal delivery of clobazam for treatment of status epileptics.
6.Holvoet C, Heyden YV, and Plaizier-Vercammen J. Inclusion complexation of lorazepam with different cyclodextrins suitable for parenteral use. Drug Dev. Ind. Pharm. 2005: 31:567– 575
7.Date AA and Nagarsenker MS. Parenteral microemulsions: an overview. Int. J. Pharm. 2008; 355:19–30.
8.Yalin M, Öner F, Öner L and Hincal AA. Preparation and properties of a stable intravenous lorazepam emulsion. J. Clin. Pharm. Ther.1997; 22:39–44.
9.Amit AK and Vandana BP. Development and Evaluation of Lorazepam Microemulsions for Parenteral Delivery. AAPS PharmSciTech, 2008; 9(3): 966-971.
10.Gladstone JP, Gawel M. Newer formulations of the triptans: advances in migraine treatment. Drugs. 2003; 63:2285Y2305.
11.Bigal ME, Bordini CA, Antoniazzi AL, Speciali JG. The triptan formulations: a critical evaluation. Arq Neuropsiquiatr. 2003; 61:313Y320.
12.Vyas TK, Babbar AK, Sharma RK Singh S and Misra A. Preliminary Brain targeting Studies on Intranasal Mucoadhesive Microemulsions of Sumatriptan AAPS PharmSciTech 2006; 7 (1) Article 8.
13.Pakalnis A, Kring D, Paolicchi J. Parenteral satisfaction with sumatriptan nasal spray in childhood migraine. J Child Neurol. 2003; 18:772Y775.
14.Villalon CM, Centurion D, Valdivia LF, de Vries P, Saxena PR. Migraine: pathophysiology, pharmacology, treatment and future trends. Curr Vasc Pharmacol. 2003;1:71Y84.
15.Martindale PK. The Complete Drug Reference. London, UK: Pharmaceutical Press; 1999:450Y452.
16.Tiwari NG and Bajaj AN. formulation development of eucalyptuss oil microemulsion for intranasal delivery. Indian J Pharm Sci 2007; 731-733.
17.Zhang Q, Jiang X, Jiang W, Lu W, Su L and Shi Z, Preparation of nimodipine loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain International Journal of Pharmaceutics, 2004; 275 (1-2, 4): 85-96.
18.Elshafeey A, Bendas E and Mohamed O. Intranasal Microemulsion of Sildenafil Citrate: in vitro evaluation and in vivo pharmacokinetic study in Rabbits, AAPS PharmSciTech 2009; 10(2):361-367.
19.Mistrya, A. Mistrya, S. Stolnika and L. Illum, Nanoparticles for direct nose-to-brain delivery of drugs, Int J Pharm, 2009; 379(1): 146–157.
20.Wu et al., H. Wu, K. Hu and X. Jiang, From nose to brain: understanding transport capacity and transport rate of drugs, Expert Opin. Drug Deliv. 2008; 5(10) : 1159–1168.
21.Westin et al., U.E. Westin, E. Boström, J. Gråsjö, M. Hammarlund-Udenaes and E. Björk, Direct nose-to-brain transfer of morphine after nasal administration to rats, Pharm. Res. 2006; 23(3): 565–572.
22.Westin et al., U. Westin, E. Piras, B. Jansson, U. Bergstrom, M. Dahlin, E. Brittebo and E. Bjork, Transfer of morphine along the olfactory pathway to the central nervous system after nasal administration to rodents, Eur. J. Pharm. Sci. 2005; 24: 565–573.
23.Borlongan and Emerich, C.V. Borlongan and D.F. Emerich, Facilitation of drug entry into the CNS via transient permeation of blood– brain barrier: laboratory and preliminary clinical evidence from bradykinin receptor agonist, Cereport. Brain Res. Bull. 2003; 60: 297–306.
24.Ying et al., W. Ying, G. Wei, D. Wang, Q. Wang, X. Tang, J. Shi, P. Zhang and H. Lu, Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia, Front. Biosci. 2007; 12: 2728–2734.
25.Wei et al., G. Wei, D. Wang, H. Lu, S. Parmentier, Q. Wang, S.S. Panter, W.H. Frey 2nd and W. Ying, Intranasal administration of a PARG inhibitor profoundly decreases ischemic brain injury, Front. Biosci. 2007; 12: 4986–4996.
26.Kumar et al., M. Kumar, A. Misra, A.K. Mishra, P. Mishra and K. Pathak, Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting, J. Drug Target. 2008; 16(10): 806–814.
27.Kumar et al., M. Kumar, A. Misra, A.K. Babbar, A.K. Mishra, P. Mishra and K. Pathak, Intranasal nanoemulsion based brain targeting drug delivery system of risperidone, Int. J. Pharm. 2008; 358(1–2): 285–291.
28.Khan et al., S. Khan, K. Patil, P. Yeole and R. Gaikwad, Brain targeting studies on buspirone hydrochloride after intranasal administration of mucoadhesive formulation in rats, J. Pharm. Pharmacol. 2009; 61(5): 669–675.
29.Vyas et al., T.K. Vyas, A.K. Babbar, R.K. Sharma, S. Singh and A. Misra, Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of Sumatriptan, AAPS PharmSciTech. 2006; 6(4): 8.
30.Huynh et al., G.H. Huynh, D.F. Deen and F.C. Szoka Jr., Barriers to carrier mediated drug and gene delivery to brain tumors, J. Control. Release 2006; 110: 236–259.
31.Hocking and Wildsmith, G. Hocking and J.A.W. Wildsmith, Intrathecal drug spread, Br. J. Anaesth. 2004; 93: 568–578.
32.Pomponi M, Giacobini E, Brufani M. Present state and future development of the therapy of Alzheimer's disease. Aging 1990;2:125-53.
33.Mygind N, Anggard A. Anatomy and physiology of the nose-pathophysiology alterations in allergic rhinnitis. Clin Rev Allergy 1984;2:173-88.
34.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci 2000;11:1-18.
35.Schipper NG, Verhoef JC, Merkus FW. The nasal mucocilliary clearance: relevance to nasal drug delivery. Pharm Res, 1991;8:807-14.
36.Mathison S, Nagilla R, Kompella UB. Nasal route for direct delivery of solutes to the central nervous system: fact or fiction? J Drug Target 1998;5:415-41.
37.Chien YW, Chang SF. Intranasal drug delivery for systemic medication. Crit Rev Ther Drug Carrier Syst, 1987;4:67-194.
38.Hehar SS, Mason JDT, Stephen AB, Washington N, Jones NS, Jackson, SJ, et al. Twenty four hour ambulatory nasal pH monitoring. Clin Otolaryngol 1999;24:24-5.
39.Chien YW, Su KSE, Chang S. Nasal systemic drug delivery. USA: Marcel Dekker; 1989. p. 1-38.
40.Reed CJ. Drug metabolism in the nasal cavity: relevance to toxicology. Drug Metab Rev, 1993;25:173-205.
41.Dahl AR, Lewis JL. Respiratory tract uptake of inhalants and metabolism of xenobiotics. Annu Rev Pharmacol Toxicol, 1993;32:383-407.
42.Thornton-Manning JR, Dahl AR. Metabolic capicity of nasal tissue interspecies comparisons of xenobiotic- metabolizing enzymes. Mutat Res, 1997;380:43-59.
43.Lewis JL, Nikula KJ, Novak R, Dahl AR. Comparative localization of carboxylesterase in F344 rat, beagle dog and human nasal tissue. Anat Rec 1994;239:55-64.
44.Aceto A, Llio, CD, Angelucci S, Longo V, Gervasi PG, Federici G. Glutathione transferases in human nasal mucosa. Arch Toxicol 1989;63:427-31.
45.Krishna NS, Getchell TV, Awasthi YC, Gatechell ML, Dhooper N. Age and gender-related trends in the expression of glutathione S-transferases in human nasal mucosa. Ann Otol Rhinol Laryngol 1995;104:812-22.
46.Ross, JS, Wilson KJW, Waugh, A, Grant, A, Ross and Wilson Anatomy and Physiology in health and illness. Churchill Livingstone; 2001. p. 206-7.
47.McMartin C, Hutchinson LE, Hyde R, Peters GE. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J Pharm Sci, 1987;76:535-40.
48.Wheatly MA, Dent J, Wheeldon EB, Smith PL. Nasal drug delivery: An in vitro characterization of transepithelial electrical properties and fluxes in the presence or absence of enhancer. J Control Release, 1988;8:167-77.
49.Tengamnuay P, Mitra AK. Transport of tyrosine and phenylalanine across the rat nasal mucosa. Life Sci, 1988;43:585-93.
50.Fisher AN, Brown K, Davis SS, Parr GD, Smith DA. The effect of molecular size on the nasal absorption of water soluble compounds in the albino rat. J Pharm Pharmacol, 1987;39:357-62.
51.McMartin, C., Hutchinson, L. E. F., Hyde, R., Peters, G. E. (1987) Analysis of Structural Requirements for the Absorption of Drugs and Macromolecules from the Nasal Cavity. J Pharm Sci, 1987; 76: 535-540.
52.Dahlin, M., Bergman, U., Jansson, B., Bjork, E., Brittebo, E. (2000) Transfer of dopamine in the olfactory pathway following nasal administration in mice. Pharml Res, 2000; 17: 737-42
53.Dahlin, M., jansson, B., Bjork, E. (2001) Levels of dopamine in blood and brain following nasal administration to rats. Eur J Pharm Sci, 2001; 14: 75-80
54.Westin, U., Piras, E., Jansson, B., Bergstrom, U., Dahlin, M., Brittebo, E., Bjork, E. Transfer of morphine along the olfactory pathway to the central nervous system after nasal administration to rodents. Eur J Pharm Sci, 2005; 24: 565
55.Miaczynska, M., Stenmark, H. Mechanisms and functions of endocytosis. Journal of Cell Biology, 180: 7-11
56.Rothen-Rutishauser, B. M., Schurch, S., Haenni, B., Kapp, N., Gehr, P. (2006) Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environmental Science & Technology, 2006; 40: 4353-4359
57.Conner, S. D., Schmid, S. L. Regulated portals of entry into the cell. Nature, 2003; 422: 37-44
58.Xiang, S. D., Scholzen, A., Minigo, G., David, C., Apostolopoulos, V., Mottram, P. L., Plebanski, M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods, 2006; 40: 1-9
59.Edeling, M. A., Smith, C., Owen, D. Life of a clathrin coat: insights from clathrin and AP structures. Nature Reviews Molecular Cell Biology, 2006; 7: 32-44
60.Sorkin, A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Current Opinion in Cell Biology, 2004; 16: 392-399
61.Anderson, R. G. W. The caveolae membrane system. Annual Review of Biochemistry, 1998; 67: 199-225
62.Frick, M., Bright, N. A., Riento, K., Bray, A., Merrified, C., Nichols, B. J. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Current Biology, 2007; 17: 1151-1156
63.Mayor, S., Pagano, R. E. Pathways of clathrin-independent endocytosis. Nature Reviews Molecular Cell Biology, 2007; 8: 603-612
64.Mygind, N., Dahl, R. Anatomy, physiology and function of the nasal cavities in health and disease. Advanced Drug Delivery Reviews, 1998; 29: 3-12
65.Gaidarov, I., Santini, F., Warren, R. A., Keen, J. H. Spatial control of coatedpit dynamics in living cells. Nature Cell Biology, 1999; 1: 1-7
66.Wearly LL. Recent progress in protein and peptide delivery by noninvasive routes. Crit Rev Ther Drug Carrier Syst, 1991;8:331-94.
67.Frey WH, Liu J, Thorne RG, Rahman YE. Intranasal delivery of 125I-labeled nerve growth factor to the brain via the olfactory route. In: Iqbal K, Mortimer JA, Winblad B, Wisniewski HM, editors. Research advances in Alzheimer's disease and related disorders. New York: John Wiley and Sons Ltd; 1995. p. 329-35.
68.Frey WH, Liu J, Chen X, Thorne RG, Fawcett JR, Ala TA. Delivery of 125I-NGF to the brain via the olfactory route. Drug Delivery 1997;4:87-92.
69.Chen XQ, Fawcett JR, Rahman YE, Ala TA, Frey WH. Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimers Dis 1998;1:35-44.
70.Frey WH, Thorne RG, Pronk G. Delivery of Insulin like growth factor-1 to the brain and spinal cord along olfactory and trigeminal pathways following intranasal administration: a noninvasive method for bypassing the blood brain barrier. Soc Neurosci Abstract 2000;26:1365-70.
71.Kucheryanu VG, Kryzhanovsky GN, Kudrin VS, Yurasov VV, Zhigaltsev IV. Intranasal fibroblast growth factors: delivery into the brain exerts antiparkinsonian effect in mice. In: Torchilin V, Veronese FM, editors. Proceedings of the 26th Internl. Symposium on controlled release of bioactive materials. Boston MA: Controlled release society Inc; 1999. p. 643.
72.Gozes I, Bardea A, Reshef A, Zamoostiano R, Zhukovsky S, Rubinraut S, et al. Neuroprotective strategy for Alzheimer disease: intranasal administration of a fatty neuropeptide. Proc Natl Acad Sci 1996;93:427-32.
73.Pietrowsky R, Struben C, Molle M, Fehm HL, Born J. Brain potential changes after intranasal administration Vs. intravenous administration of vasopressin: evidence for a direct nose-brain pathway for peptide effects in humans. Biol Psychiatry 1996;39:332-40.
74.Pietrowsky R, Thieman A, Kern W, Fehm HL, Born JA. A nose-brain pathway for psychotropic peptides: evidence from a brain evoked potential study with cholecystokinin. Psychoneuroendocrinology 1996;21:559-72.
75.Smolnik R, Molle M, Fehm HL, Born J. Brain potentials and attention after acute subchronic Intranasal administration of ACTH4-10 desacetyl-a-MSH in humans. Neuroendocrinol 1999;70:63-72.
76.Fehm HL, Smolnik R, Kern W, McGregor GP, Bickel U, Born J. The melanocortin melanocyte-stimulating hormone/adrenocotropin (4-10) decreases body fat in humans. J Clin Endocrinol Metab 2001;86:1144-8.
77.Kern W, Born J, Schreiber H, Fehm HL. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes 1999;48:557-63.
78.Hinchcliffe M, Illum L. Intranasal insulin delivery and therapy. Adv Drug Deliv Rev 1999;35:199-234.
79.Hussain AA. Intranasal drug delivery. Adv Drug Deliv Rev 1998;29:39-49.
80.Agarwal V, Mishra B. Recent trends in drug delivery systems: intranasal drug delivery. Indian J Exp Biol 1999;37:6-16.
81.Schipper NGM, Verhoef JC, Merkus FWHM. Methylated beta-cyclodextrin is able to improve the nasal and intramuscular administration of salmon calcitonin. Calcif Tissue Int 1995;56:280-2.
82.Illum L, Dodane, V, Iqbal, K. Chitosan technology to enhance the effectiveness of nasal drug. Drug Del Technol 2002;2:1-7.
83.Illum L. Animal models for nasal delivery. J Drug Target 1996;3:717-24.
84.Illum L. Bioadhesive formulations for nasal peptide delivery. In: Mathiowitz E, Chickering DE, Lehr C, editors. Bioadhesive drug delivery systems. USA: Marcel Dekker; 1999. p. 507-41.
85.Illum L. Chitosan and its use as pharmaceutical excipient. Pharm Res 1998;15:161-70.
86.Illum L, Watts P, Fisher AN, Gabble-gill, Davis SS. Novel chitosan based delivery systems for nasal administration of a LHRH-analogue. STP Pharma 2000;10:89-94.
87.Illum L, Fisher AN, Gabble-gill I, Davis SS. The effect of bioadhesive starch microsphere on the absorption enhancing effect of enhancing agents administered nasally. Int J Pharm 2001;222:109-19.
88.Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH. Intranasal administration of insulin-like growth factor-I bypass the blood brain barrier and protects against focal cerebral ischemic damage. J Neuro Sci 2001;187:91-7.
89.Liu XF, Fawcett JR, Thorne RG, Frey WH 2nd. Non-invassive intranasal insulin-like growth factor-I reduces infarct volume and improves neurologic function in rats following middle cerebral artery occlusion. Neurosci Lett 2001;308: 91-4.
90.Liu XF, Fawcett JR, DeFor TA, Frey W. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive Intranasal administration of Insulin-like growth factor-I. J Stroke Cerebrovascular Dis 2001;10:194-8.
91.Gozes I, Giladi E, Pinhasov A, Bardea A, Brenneman DE. Activity-dependent neurotrophic factor: Intranasal administration of femotmolar-acting peptides improve performance in a water maze. J Pharmacol Exp Ther 2000;293:1091-8.
92.Kern W, Schiefer B, Schwarzenburg J, Stange EF, Born J, Fehm HL. Evidence for central nervous effects of corticotropin-releasing hormone on gastric acid secretion in humans. Neuroendocrinology 1997;65:291-8.
93.Perras B, Marshall L, Kohler G, Born J, Fehm HL. Sleep and endocrine changes after intranasal administration of growth hormone-releasing hormone in young and aged humans. Psychoneuroendocrinology 1999;24:743-57.
94.Al-Ghananeem AM, Traboulsi AA, Dittert LW, Hussain AA. Targeted brain delivery of 17 -estradiol via nasally administered water soluble prodrugs. AAPS Pharm Sci Tech 2002;3:1-8.
95.Stratford RE Jr, Lee VH. Aminopeptidase activity in homogenates of various absorptive mucosa in albino rabbit : Implication in peptide delivery. Int J Pharm 1986;30:73-82.
96.Vajdy M, O'Hagan DT. Microparticles for intranasal immunization. Adv Drug Deliv Rev 2001;51:127-41.
97.Oh YK, Kim JP, Hwang TS, Ko JJ, Kim JM, Yang JS, et al. Nasal absorption and biodistribution of plasmid DNA: an alternative route of DNA vaccine delivery. Vaccine 2001;19:4519-25.
98.Anand Kumar TC, David GFX, Umberkoman B, Saini KD. Uptake of radioradioactivity by body fluids and tissues in rhesus monkeys after intravenous injection or intranasal spray of tritium-labelled estradiol and progesterone. Curr Sci 1974;43:435-9.
99.David GF, Puri CP, Anand Kumar TC. Bioavailability of progesterone enhanced by intranasal spraying. Experentia 1981;37:533-4.
100.Sippel JM, Giraud GD, Holden WE. Nasal administration of the nitric oxide synthase inhibitor L-NAME-induces daytime somnolence. Sleep 1999;22:786-8.
101.Chow HS, Chen Z, Matsuura GT. Direct transport of cocaine from the nasal cavity to the brain following intranasal cocaine administration in rats. J Pharm Sci 1999;88:754-8.
102.Sakane T, Akizuki M, Yamashita S, Nadai T, Hashida M, Sezaki H. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the dissociation of the drug. J Pharm Pharmacol 1994;46:378-9.
103.Sakane T, Akizuki M, Yamashita S, Nadai T, Hashida M, Sezaki H. The transport of a drug to the cerebrospinal fluid from the rat nasal cavity: the relation to the lipophilicity of the drug. Chem Pharm Bull 1991;39:2456-8.
104.Sakane T, Akizuki M, Yamashita S, Nadai T, Hashida M, Sezaki H. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the molecular weight of drugs. J Pharm Pharmacol 1995;47:379-81.
105.Sakane T, Akizuki M, Yamashita S, Nadai T, Hashida M, Sezaki H. The transport of cephalexin to the cerebrospinal fluid directly from the nasal cavity. J Pharm Pharmacol 1991;43:449-51.
106.Dahlin M, Jansson B, Bjork E. Levels of dopamine in blood and brain following nasal administration to rats. Eur J Pharm Sci 2001;14:75-80.
107.Dahlin M, Bergman U, Jansson B, Bjork E, Brittebo E. Transfer of dopamine in the olfactory pathway following nasal administration in mice. Pharm Res 2000;17:737-42.
108.Ishikawa F, Katsura M, Tamai I, Tsuji A. Improved nasal bioavailability of elcatonin by insoluble powder formulation. Int J Pharm 2001;224:105-14.
109.Bergstrom U, Franzen A, Eriksson C, Lindh C, Brittebo EB. Drug targeting to brain: transfer of picolinic acid along the olfactory pathways. J Drug Target 2002;10:469-78.
110.Hussain A, Dittert LW, Traboulsi A. Brain delivery of folic acid for the prevention of Alzheimer's disease and stroke. US: Patent 6; 2002. p. 369,058.
111.Li L, Nandi I, Kim KH. Development of an ethyl laurate-based microemulsion for rapid-onset intranasal administration of diazepam. Int J Pharm 2002;237:77-85.
112.Illum L, Watts P, Fisher AN, Hinchcliffe M, Norbury H, Jabbal-Gill I, et al. Intranasal delivery of morphine. J Pharmacol Exp Ther 2002;301:391-400.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









