{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Mir Sahidul Ali1, Souvik Mukherjee2, Debayan Roy2, Gargi Pal3, Subhajit Makar*2
1Department of Chemical & Polymer Engineering, Tripura University (A Central University), Suryamaninagar, Tripura 799022, India.
2School of Basic & Applied Sciences, Central University of Punjab,Bathinda-155110, Punjab, India.
3 Department of Pharmaceutical Sciences and Technology, NSHM Knowledge Campus, Kolkata- 700053, India.
ABSTRACT: Asparagus racemosus (A. racemosus) is a well-known medicinal plant due its various application in Ayurveda. It contains various type of secondary metabolite active natural products (or ingredients) such as steroids, alkaloids, flavonoids, furan derivatives and essential oils. In the present scenario due to growing catastrophic effect of diseases in human life, researchers are trying to stop synthetic drugs by replacing with herbal drugs. Herbal drug treatments are in general used to provide first-line and public health provider, both to persons abode in faraway areas the place it is the only on hand wellness service, and to people residing in bad areas the place it offers the one cheap relief. In this point of view A. racemosus can show a new path of herbal treatment.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2630
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 12 Received On: 31/10/2018; Accepted On: 17/11/2018; Published On: 01/12/2018 How to cite this article: Makar, S., Ali, M.S., Mukherjee, S., Roy, D. and Pal, G. 2018. Asparagus Racemosus, a Climbing Ayurvedic Medicinal Plant: Review on its Cultivation, Morphology and Medicinal Significance. PharmaTutor. 6, 12 (Dec. 2018), 46-54. DOI:https://doi.org/10.29161/PT.v6.i12.2018.46 |
INTRODUCTION:
Medicinal plants are the nature’s gift to human being to cure disease and make healthy life. Herbal medicine is still the backbone of about 75-80% of the total population, mainly in developing nations, for primary health care because, better compatibility with the human body and fewer side effects (Barmet, 1992). In India thousands of species are known to have medicinal importants and the use of different parts of several medicinal plants to cure specific ailments has been in trend since ancient times (Parekh, Jadeja, & Chanda, 2006). Herbal medication, also called botanical treatment or phytomedicine, denotes to the use of any plant's parts such as seeds, berries, roots, leaves, bark, or flowers for medicinal purposes. Shatavari means who hold potential to cure “hundred diseases or suitable to many” (Bopana & Saxena, 2007). Asparagus racemosus (Shatavari) is encouraged in Ayurvedic texts for the prevention and medication of gastric ulcers, dyspepsia and as a galactogogue. A. racemosus has additionally been used by some Ayurvedic practitioners for worried problems. It is considered as huge efficient tonic both for male and female for fertility from long time. Due to its remarkable Pharmacological activity, in Ayurveda Sastra it is known as “Queen of herbs” Shatavari is known in various name in various part of India and also around the globe. . Use of A. racemosus is vindicated in ancient literature of Ayurveda (Charaka Samhita)(Chawla, Chawla, Mangalesh, & Roy, 2011). Its medicinal uses has been reported in the Indian Pharmacopoeias and British Pharmacopoeias and in traditional system of medicine such as Ayurveda, Unani and Siddha. In recent years, a large number of plant products have been inspected for their antimicrobial stuffs against bacteria and fungi. The study will also confirm if there is a biological basis to the claim that the ethnomedicinal plant has useful medicinal purposes (Cowan, 1999).
The genus Asparagus existed around 300 species around the world, out of which 22 species found in India. A. racemosus is broadly distributed around various corner of the world.(Kirtikar, 1918). Medicinal plants and herbal drugs account for a giant percent of the pharmaceutical market (Hawkins & Ehrlich, 2007)
Plant Characteristics:
Kingdom : Plantae
Clade : Angiosperms
Clade : Monocots
Order : Asparagales
Family : Asparagaceae
Sub family : Asparagoideae
Genus : Asparagus
Species : Asparagus racemosus L
Various common names at various region:
Sanskrit : Satavari
Hindi : satavari, shatawar
Bengali : Shatamuli
Tamil : Inli-Chedi
Telegu : Pilli-gaddalu
Kumaon : kairuwa
Rajasthani: Norkanto or Satawar
Gujrati : Satawari
Nepali : Kurilo
CULTIVATION, CHARACTERISTICS AND MORPHOLOGY:
It was first botanically defined in 1799(GRIN). It grows one to two meters tall and desires to take root in raspy, stony soils high up in piedmont plains, at height 1300-1400 m altitude (Freeman, 2009).
Cultivation:
A. racemosus is a common and important medicinal plant which grow up all over the India, Sri Lanka, and Himalayas & over the World. Due to its various medicinal application the demand of A. racemosus is constantly increases, because of destructive harvesting, combined with habitat destruction and deforestation this plant fall in a risk situation. In Thailand roots of the tree have been used for treatment of spleen, liver and other internal organs (Wiboonpun, Phuwapraisirisan, & Tip‐pyang, 2004). In India, generally roots are utilized as a remedy for internal pains, tumors, fever and as a tonic (Kala, 2009).

Figure 1. Pictures of A. Racemosus Showing Flowers, Leaves, Fruits, Tuberous Roots
Characteristics:
A. racemosus is a woody climbing plant growing to 1-2 m in length. The leaves are small like pine needles, uniform and shiny green, flowers are white and have small spikes and in September month it fruits, producing blackish purple like globular shapes.
Economic Importance: Use as Medicines (Arnold & De Wet, 1993)
Habitat:
• Africa:
1. East Tropical Africa: Kenya, Tanzania
2. Northeast Tropical Africa: Ethiopia, Somalia, Sudan
3. East Tropical Africa: Angola, Mozambique
4. Southern Africa: Namibia, Limpopo, Swaziland
• Asian country:
India, Nepal, Bhutan, Pakistan, Sri Lanka, Myanmar, Malaysia
• Australasia:
Australia Queensland, Western Australia, Northern Territory
Table 1: traces elements (alkaline earth metals & transition metals) extract from Asparagus Racemosus.
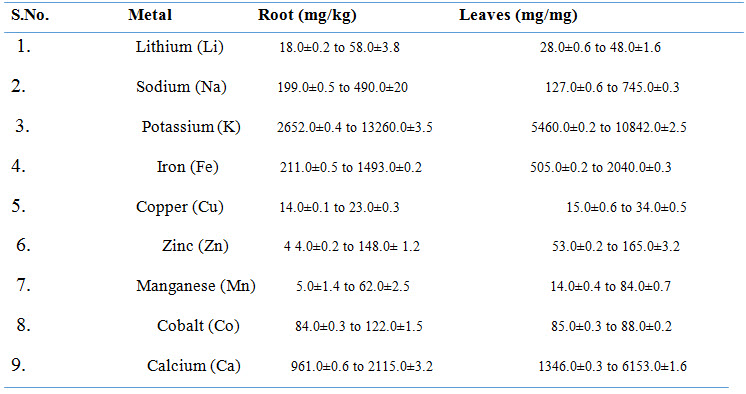
Phytochemicals:
A. racemosus consists of a diverse range of bio active and medicinally importance molecules in which major constituent is steroidal saponins along with alkaloids, flavonoids, dihydrophenanthrene derivatives, furan derivatives (table 1) and volatile constituents (figure 2-3). Twenty nine steroidal saponins were reported from A.Racemosus. An oligospirostanoside named 3-0-[α-Lrhamnopyranosyl-(1→2)-α-L-rhamnopyranosyl(1→4)-O-β-D-glucopyranosyl]-25(S)-spirosta-3βoil is obtained from A. racemosus which on oral administration potentiated antibody synthesis and enhanced cell-mediated immune response in immune cooperated animals (Handa et al., 2003).
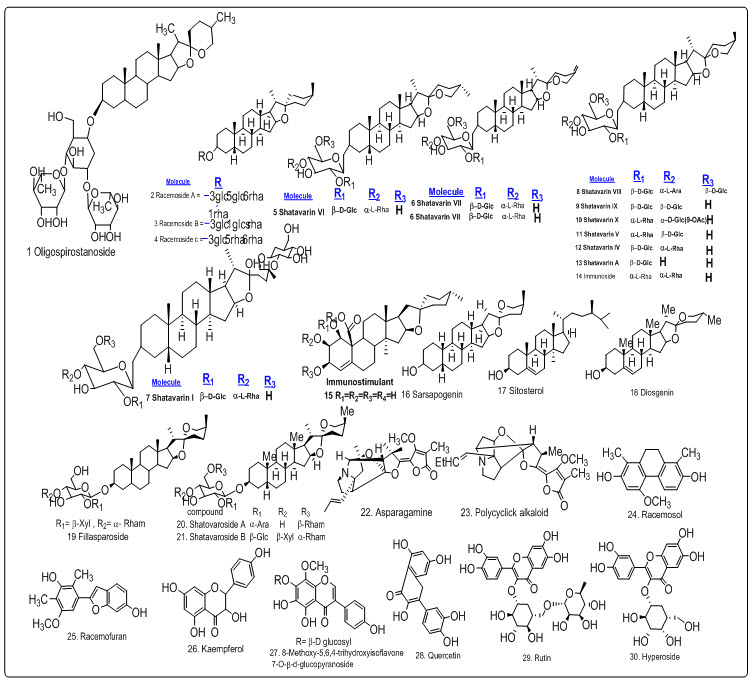
Fig.2 Structures of steroidal saponins, alkaloids, dihydrophenanthrene, furan derivative, flavonoids obtained from A. Racemosus
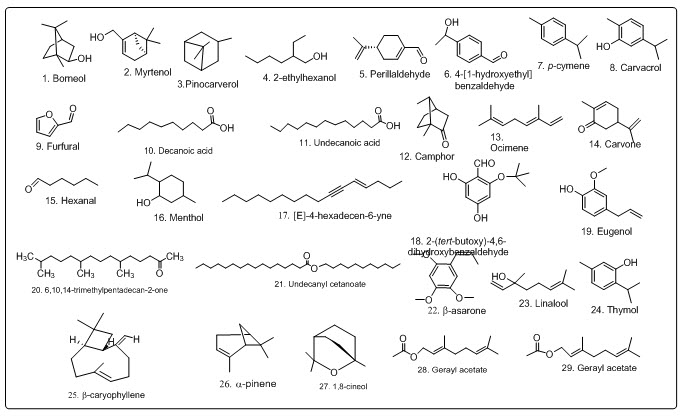
Fig.3 Structures of volatile components of A. Racemosus
Medicinal Significance:
Asparagus racemosus could be a principal healthful plant of tropical and semitropical India. Its healthfulutilization has been pronounced within the Indian and British formulary it's very often known for its phytoestrogenic residences. In written material, Asparagus racemosus has been delineated as a rasayana herb and has been used unremarkably as an adaptogen to expand the non-distinctive resistance of organisms against a spread of stresses except use inside the healing of symptom and infectious disease, the plant additionally has inhibitor, immunostimulant, antidyspepsia and medicament results. The roots area unit usedin Ayurvedic medication, following a programme of process and drying. It's commonly used as a female internal reproductive organ tonic, as agalactogogue (to provides a boost to breast milk), in acidity, and as a best common wellbeing tonic (Nag, Mukherjee, Roy, Paul, & Sahidul). A. racemosus is a outdated galactogogue both in animals and human’s therapy of duodenal ulcer with A. racemosus was mentioned. Antioxitocin action of this plant had been recognized and immunomodulatory properties are ascribed to the basis. The presence of antifungal and antimicrobial compounds in the higher plants is well established as they have delivered a source of inspiration for novel drug compound as plant derived medicines have made significant contribution towards human the treatment of diseases as is done in cases of Unani and Ayurvedic system of medicines.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
1. Antimicrobial activity:
The bacterial organisms including Gram positive and Gram negative like different species of Bacillus, Staphylococcus, Salmonella and Pseudomonas are the main source to cause severe infections in humans. Because these organisms have the ability to survive in harsh condition due to their multiple environmental habitats (Ahameethunisa & Hopper, 2010). In the worldwide as well as in the developing countries, the most human died due to infectious bacterial diseases (Nathan, 2004). The various inorganic and organic solvent extract of A. Racemosus show anti-bacterial and anti-fubgal activity (Patel & Patel, 2013). The root extracts of A. racemosus have been investigated for antibacterial property employing standard cylinder method. Microbes used were Bacillus subtilis, Staphylococcus aureus, Staphylococcus werneri, Pseudomonas aeruginosa and Escherichia coli, Proteus mirabilis, Kleibsella pneumonia, Pseudomonas putida. Both grampositive and gram-negative bacteria were sensitive to the extract. Ethanolic extract of concentration100mg/ml, 300mg/ml, 500mg/ml were prepared and there antibacterial activity was comparable to reference standard drug Gentamycin (25μg), Staphylococcus aureus the gram positive bacteria were most affected (Ravishankar et al., 2012). Experimental findings suggested that methanol extracts possessed high anticandidal activity against different Candida species. The MIC (Minimum inhibitory concentration) values were between 2.5 to 0.312 mg/ml, while MFC (Minimum fungicidal concentration) values ranged between 5 to 0.625 mg/ml (Uma, Prabhakar, & Rajendran, 2009).
2. Anticancer activity:
Cancer is a deadly disease of striking significance in the world today. It is one of the most serious health threats, responsible for the death of about seven million people in a year and a great challenge to medical science (Makar, Saha, & Singh, 2018). The root extract was shown to possess a protecting impact within the duct gland cell cancer eighty. endocrineelements of the A. racemosus were investigated for the apoptotic activity and inferred to possess the capabilityto tumour necrobiosis (Bhutani, Paul, Fayad, & Linder, 2010). Anticancer property of shatavarins (containing Shatavarin IV) which was extract from the roots of have been evaluated by MTT assay using MCF-7 (human breast cancer), HT-29 (human colon adenocarcinoma) and A-498 (human kidney carcinoma) cell lines and in vivo experimental model of Ehrlich ascites carcinoma (EAC) tumor bearing rats (Mitra, Prakash, & Sundaram, 2012). An aqueous solution of the crude alcoholic extract of the roots extract also shows antiprotozoal activity (Alok et al., 2013).
3. Antidepressant activity:
Adaptogenic drugs are such drugs which are useful as antistress agents by enhancing non-specific resistance of the body. Although, the adaptogenic effect of A. racemosus is well documented, its use in psychological disorders like depression is not scientifically evaluated. Hence, the present investigation evaluates the antidepressant effect of MAR standardized to saponins (62.2% w/w). Rats were given methanolic extract of roots of A. racemosus in doses of 100, 200 and 400 mg/kg every day for 7 day and then subjected to forced swim test (FST) and learned helplessness test (LH). The results showed that MAR decreased immobility in FST and increased avoidance response in LH indicating antidepressant activity (Singh, Garabadu, Muruganandam, Joshi, & Krishnamurthy, 2009).
4. Antiurolithiatic activity:
The mice treated with ethanolic extract of A. racemosus at doses 800 and 1 600 mg/kg significantly (P<0.05) decreased the serum concentrations of calcium, phosphorus, urea and creatinine (Jagannath, Chikkannasetty, Govindadas, & Devasankaraiah, 2012).
5. Diuretic activity:
Critical toxicity study revealed no fatality even with the highest dose, and the diuretic study revealed significant diuretic activity in dose of 3 200 mg/kg (Kumar et al., 2010).
6.Cardiovascular effects:
Abana, herbomineral formulation maketed by Himalaya Drugs Company, have been found beneficial for controlling hypercholesterolemia, prevention and management of coronary heart disease. The lipid-lowering capability of A. racemosus root extract in hypercholesteremic mice was verified and the investigation revealed that primary reason of antihypercholestrolemic effect was increased excretion of cholesterol, neutral sterols, bile acid and increase in hepatic bile acid content (Tiwari, Agarwal, Shukla, & Dubey, 1990).
7. Antineoplastic activity:
Chloroform-methanol (1:1) extract of fresh root of A. racemosus has been reported to reduce the tumor case in female rats treated with 7, 12 dimethyl benza. This exploit is suggested to be medicated by virtue of mammotropic and/ or lactogenic (Rao, 1981).
8.Effect on central nervours system:
Neither stimulant nor depressant action of lactare on central nervous system (CNS) have been reported in albino mice. Shatavari did not produce catalepsy in experimental rats, even with massive oral doses, telling that its action may be outside the blood-brain barrier (BBB), similar to that of metoclopramide (Parihar & Hemnani, 2004).
9. Anti-inflammatory effects:
ACE inhibited topical edema in the mouse ear, following supervision at 200 mg/kg (i.p.), important to substantial reductions in skin thickness and tissue weight, inflammatory cytokine production, neutrophil-mediated myeloperoxidase activity, and various histopathological indicators. Furthermore, ACE was fruitful at reducing inflammatory damage induced by chronic TPA exposure and evoked a significant inhibition of vascular permeability influence by acetic acid in rat (Choo et al., 2009).
10. Anti-HIV effects:
A. racemosus is also known to show immunomodulatory activity. Steroidal saponin glycosides have been reported from these extracts. Compound 19 isolated from the ethanolic extract exhibited the highest anti-HIV activity as compared to other saponin glycosides 35. Glycoside 20 with two sugars exhibited weak anti-HIV activity.

Fig.4 SAR studies of anti-HIV chemical constitutes of A.racemosus.
11. Antihepatotoxic activity:
Ethanolic extract of root of A. racemosus has been exposed to significantly decrease the enhanced levels of alanine transakinase, aspartate transaminase and alkaline phosphate in carbon tetra chloride (CCl 4 ) induced heptic damagein rats, indicating antihepatotoxic potential of A. Racemosus (Zhu, Zhang, Zhao, Wang, & Qu, 2010).
12. Gastrointestinal effects:
The dried crushed root of A. racemosus is used in Ayurveda for dysepesia. Oral administration of powdered dried root of A. racemosus has been found to stimulate gastric emptying in healthy volunteers. Its action is reported to be comparable with that of the synthetic dopamine antagonist metoclopramide (Dalvi, Nadkarni, & Gupta, 1990). In Ayurveda, A. racemosus has also been mentioned for the treatment of ulcerative disorder of stomach and parinama sula, a clinical object akin to the duodenal ulcer disease
13. Antioxidant effects:
The probable antioxidant activity of crude extract and purified water fraction of A. racemosus against member injury induced by the free radicals generated during gama (γ) radiation were studied in rat liver mitochondria. Using an effective dose of 450 Gray antioxidant effect of A. racemosus extract were studied against oxidative damage term of protection against lipid peroxidation, protein oxidation. cantly inhibited lipid peroxidation and protein oxidation. The antioxidant activity of P3 fraction was more distinct against lipid peroxidation, as assessed by thiobarbituric acid reactive substance generation, while that of crude extract was more active in inhibiting proteins oxidation (Visavadiya, Soni, & Madamwar, 2009).
14. Teratogenicity effects:
Shatvari is a herb used as a rasayna in Indian Ayurveda and is considered both male and female reproduction tonic. Methanolic extract of A. racemosus roots (MAR), 100 mg/kg per day for 60 d, showed teratological disordres in terms of increase resorption of fetuses, gross malformation e.g. swelling in legs and intrauterine growth. A. racemosus should be used pregnancy constantly as its exposure during that period may cause damage to the offspring (Goel, Prabha, Kumar, Dorababu, & Singh, 2006).
15. Increase memory and protects against amnesia:
MAR also significantly reversed scopolamine and sodium nitrite-induced increase in transfer latency on elevated plus maze indicating anti-amnesic activity. Further, MAR dose-dependently inhibited acetylcholinesterase enzyme in specific brain regions (prefrontal cortex, hippocampus and hypothalamus). Thus, MAR showed nootropic and anti-amnesic activities. A. racemosus extract demonstrated significant decrease in latency time during retention trials. A. racemosus can be recognized to their antioxidant, neuroprotective and cholinergic properties (Ojha, Sahu, Muruganandam, Singh, & Krishnamurthy, 2010)
16. Immunostimulant activity:
Intra-abdominal sepsis is key causes of mortality following trauma and bowel surgery. . Immunomodulating property of A. racemosus has been revealed to defend the rat and mice against experimental induced abdominal sepsis (Thatte, Chhabria, Karandikar, & Dahanukar, 1987). Oral administration of decotion of powdered root of A. racemosus been reported to produce leucocytosis and predominant neutrophilia along with greater phagocytic activity of the macrophages and polymorphs.
17. Miscellaneous effects:
Alcoholic extract of root of A. racemosus was found to have trivial diuretic effect in rats and hypoglycemic action in bunny, but no anticonvulsant and antifertility effect was observed in rats and rabbits, respectively. However, it did show some antiamoebic effect in rats.
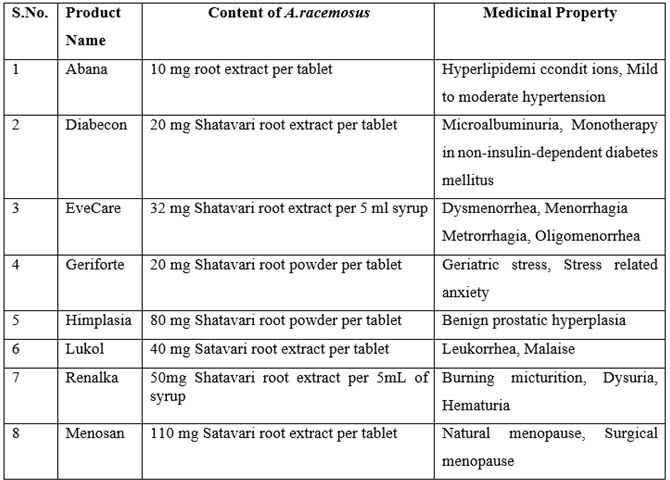
Marketed Drugs:
Table no.2 List of Marketed drugs contain A. Racemosus
CONCLUSION: A. Racemosus is an important medicinal plant which used from ancient time in India, this review paper could be an answer to the people seeking for better therapeutic agents from natural sources which is believed to be more efficient with lesser or no side effects when compared to the commonly used synthetic agents.
REFERENCES:
1. Ahameethunisa, A. R., & Hopper, W. (2010); Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria; BMC complementary and alternative medicine, 10(1), 6.
2. Alok, S., Jain, S. K., Verma, A., Kumar, M., Mahor, A., & Sabharwal, M. (2013); Plant profile, phytochemistry and pharmacology of Asparagus racemosus (Shatavari): A review; Asian Pacific journal of tropical disease; 3(3); 242-251
3. Arnold, T. H., & De Wet, B. (1993). Plants of southern Africa: names and distribution: National Botanical Institute.
4. Barmet, H. (1992); The natural pharmacy: An encyclopedic illustrated guide to medicine from nature. Mirriampolunin and Christopher Robins, Great Britain.
5. Bhutani, K., Paul, A., Fayad, W., & Linder, S. (2010); Apoptosis inducing activity of steroidal constituents from Solanum xanthocarpum and Asparagus racemosus. Phytomedicine, 17(10), 789-793.
6. Bopana, N., & Saxena, S. (2007); Asparagus racemosus—Ethnopharmacological evaluation and conservation needs; Journal of ethnopharmacology, 110(1), 1-15.
7. Chawla, A., Chawla, P., Mangalesh, P., & Roy, R. (2011); Asparagus racemosus (Willd): biological activities & its active principles; Indo Global J Pharm Sci; 1(2); 113-120.
8. Choo, B. K., Yoon, T., Cheon, M. S., Lee, H. W., Lee, A. Y., & Kim, H. K. (2009). Anti-inflammatory effects of Asparagus cochinchinensis extract in acute and chronic cutaneous inflammation. Journal of ethnopharmacology, 121(1), 28-34.
9. Cowan, M. M. (1999); Plant products as antimicrobial agents. Clinical microbiology reviews; 12(4); 564-582.
10. Dalvi, S., Nadkarni, P., & Gupta, K. (1990); Effect of Asparagus racemosus (Shatavari) on gastric emptying time in normal healthy volunteers; Journal of Postgraduate Medicine, 36(2), 91-94
11. Freeman, R. (2009). Liliaceae-famine foods. Centre for New Crops and Plant Products, Department of Horticulture & Landscape Architecture. Purdue University. Retrieved April, 25.
12. Goel, R., Prabha, T., Kumar, M. M., Dorababu, M., & Singh, G. (2006). Teratogenicity of Asparagus racemosus Willd. root, a herbal medicine.
13. Handa, S. S., Suri, O. P., Gupta, V. N., Suri, K. A., Satti, N. K., Bhardwaj, V., . . . Parikh, G. G. (2003). Process for the isolation of novel oligospirostanoside: Google Patents.
14. Hawkins, E., & Ehrlich, S. (2007). Herbal Medicine: Overview.
15. Jagannath, N., Chikkannasetty, S. S., Govindadas, D., & Devasankaraiah, G. (2012); Study of antiurolithiatic activity of Asparagus racemosus on albino rats; Indian journal of pharmacology, 44(5); 576-579
16. Kala, C. P. (2009); Aboriginal uses and management of ethnobotanical species in deciduous forests of Chhattisgarh state in India. Journal of Ethnobiology and Ethnomedicine; 5(1); 20.
17. Kirtikar, K. R. (1918). Indian Medicinal Plants Vol-4: Bishen Singh Mahendra Pal Singh; Dehradun.
18. Kumar, M., Udupa, A., Sammodavardhana, K., Rathnakar, U., Shvetha, U., & Kodancha, G. P. (2010); Acute toxicity and diuretic studies of the roots of Asparagus racemosus Willd in rats. West Indian Medical Journal; 59(1); 03-06.
19. Makar, S., Saha, T., & Singh, S. K. (2018); Naphthalene, a versatile platform in medicinal chemistry: Sky-high perspective. European Journal of Medicinal Chemistry.
20. Mitra, S. K., Prakash, N. S., & Sundaram, R. (2012); Shatavarins (containing Shatavarin IV) with anticancer activity from the roots of Asparagus racemosus. Indian journal of pharmacology; 44(6); 732-736
21. Nag, M., Mukherjee, S., Roy, D., Paul, R. K., & Sahidul, M. Extraction and Chemical tests on Cicer Arietinum seed collected from North Bengal Region of West Bengal, India.
22. Nathan, C. (2004); Antibiotics at the crossroads; Nature, 431(7011), 899.
23. Ojha, R., Sahu, A. N., Muruganandam, A., Singh, G. K., & Krishnamurthy, S. (2010);Asparagus recemosus enhances memory and protects against amnesia in rodent models. Brain and cognition;74(1);1-9.
24. Parekh, J., Jadeja, D., & Chanda, S. (2006); Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turkish Journal of Biology, 29(4); 203-210.
25. Parihar, M., & Hemnani, T. (2004); Experimental excitotoxicity provokes oxidative damage in mice brain and attenuation by extract of Asparagus racemosus; Journal of Neural Transmission, 111(1); 1-12.
26. Patel, L., & Patel, R. (2013); Antimicrobial Activity of Asparagus Racemosus Wild From Leaf Extracts–a Medicinal Plant. International Journal of Scientific and Research Publications; 3(3); 2250-3153.
27. Rao, A. (1981); Inhibitory action of Asparagus racemosus on DMBA‐induced mammary carcinogenesis in rats; International journal of cancer; 28(5); 607-610.
28. Ravishankar, K., Kiranmayi, G., Lalitha, T. M., Priyanka, T., Ranjith, T., Someswarao, S., . . . Divya, A. (2012); Preliminary phytochemical screening and in vitro antibacterial activity on Asparagus racemosus root extract; Int J Pharm Chem Biol Sci, 2(1); 117-123.
29. Singh, G. K., Garabadu, D., Muruganandam, A., Joshi, V. K., & Krishnamurthy, S. (2009); Antidepressant activity of Asparagus racemosus in rodent models. Pharmacology Biochemistry and Behavior; 91(3); 283-290.
30. Thatte, U., Chhabria, S., Karandikar, S., & Dahanukar, S. (1987); Immunotherapeutic modification of E. coli induced abdominal sepsis and mortality in mice by Indian medicinal plants. Indian drugs, 25(3); 95-97.
31. Tiwari, A., Agarwal, A., Shukla, S., & Dubey, G. (1990); Favourable effect of Abana on lipoprotein profiles of patients with hypertension and angina pectoris. Alternative Med, 3(3) ; 139-142.
32. Uma, B., Prabhakar, K., & Rajendran, S. (2009); Anticandidal activity of Asparagus racemosus; Indian journal of pharmaceutical sciences, 71(3); 342-343
33. Visavadiya, N., Soni, B., & Madamwar, D. (2009). Suppression of reactive oxygen species and nitric oxide by Asparagus racemosus root extract using in vitro studies. Cellular and molecular biology (Noisy-le-Grand, France), 55, OL1083-1095.
34. Wiboonpun, N., Phuwapraisirisan, P., & Tip‐pyang, S. (2004); Identification of antioxidant compound from Asparagus racemosus. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives; 18(9); 771-773.
35. Zhu, X., Zhang, W., Zhao, J., Wang, J., & Qu, W. (2010); Hypolipidaemic and hepatoprotective effects of ethanolic and aqueous extracts from Asparagus officinalis L. by‐products in mice fed a high‐fat diet; Journal of the Science of Food and Agriculture, 90(7);1129-1135.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









