ABOUT AUTHORS:
Rituraj Singh Chundawat*, Yuvraj Singh Sarangdevot, Bhupendra Vyas, Gajendra Singh Rathore, Udaibhan Singh Rathore, Pankaj Sharma
Department of Quality Assurance, Bhupal Nobles’ College of Pharmacy,
Udaipur- 313002, Rajasthan, India
*riturajsingh.chundawat@gmail.com
ABSTRACT
Pharmaceutical analysis may be defined as a process or a sequence of processes to identify and/or quantify a substance or drug, the components of a pharmaceutical solution or mixture or the determination of the structures of chemical compounds used in the formulation of pharmaceutical product. HPLC is the most rapid and reliable analytical technique for analysis of drugs. Its simplicity, high specificity and wide range of sensitivity makes it ideal for analysis of many drugs in both dosage forms and biological fluids. Montelukast is a CysLT1 antagonist which blocks the action of leukotriene D4 (and secondary ligands LTC4 and LTE4) on the cysteinyl leukotriene receptor CysLT1 in the lungs and used in asthma. Fexofenadine competes with free histamine for binding at H1-receptors in the GI tract, large blood vessels, and bronchial smooth muscle. It blocks the action of endogenous histamine and relieves from allergy. The analytical method development of Montelukast and Fexofinadine combination in pharmaceutical dosage form is done by using HPLC method.
REFERENCE ID: PHARMATUTOR-ART-1761
1. INTRODUCTION
1.1 Introduction to Drug Analysis
Pharmaceutical analysis is a branch of practical chemistry that involves a series of process for identification, determination, quantification and purification of a substance, separation of the components of `a solution or mixture, or determination of structure of chemical compounds. The substance may be a single compound or a mixture of compounds and it may be in any of the dosage form. The substance used as pharmaceuticals are animals, plants, micro organisms, minerals and various synthetic products. Analytical determination is based on the measurement of some physical, Chemical or structural properties which are related directly or indirectly to the amount of desired constituent present in the sample. Drugs or pharmaceuticals are chemicals which are of organic, inorganic or other origin. Whatever may be the origin, some property of the medicinal agent is used to measure them qualitatively and quantitatively.e.g. Gravimetric analysis, Acidimetry and Alkalimetry Methods.
Introduction to selection of Analytical Method
A vital first step in any quantitative analysis is the selection of method. This will require careful consideration of following criteria.
- The type of analysis required.
- Problems arising from the nature of material to be investigated.
- Possible interference from components of the material other than those of interest.
- The concentration range, which needs to be investigated.
- The accuracy required.
- The facilities available.
- The time required to complete the analysis.
Finally, the choice of method is always governed by complexity of sample as well as number of components in the samples.
Analytical methods are generally classified into two categories
- Instrumental
- Non-instrumental
1.3 Analytical Method Development
1.3.1 Introduction
The number of drugs introduced into the market is increasing every year. These drugs may be either new entities or partial structural modification of the existing one. Very often there is a time lag from the date of introduction of a drug into the market to the date of its inclusion in pharmacopoeias.
1.3.2 Basic criteria for new method development of drug analysis:
- The drug or drug combination may not be official in any pharmacopoeias,
- A proper analytical procedure for the drug may not be available in the literature due to patent regulations,
- Analytical methods may not be available for the drug in the form of a formulation due to the interference caused by the formulation excipients,
- Analytical methods for the quantitation of the drug in biological fluids may not be available,
- Analytical methods for a drug in combination with other drugs may not be available,
- The existing analytical procedures may require expensive reagents and solvents. It may also involve cumbersome extraction and separation procedures and these may not be reliable.
1.4 Introduction to HPLC
1.4.1 Introduction
HPLC is fastest growing analytical technique for analysis of drugs. Its simplicity, high specificity and wide range of sensitivity makes it ideal for analysis of many drugs in both dosage forms and biological fluids.
HPLC is presently used in pharmaceutical research and development in following areas:
1) To purify synthetic or natural products.
2) To characterize metabolites.
3) To assay active ingredients, impurities, degradation products and in dissolution studies.
4) In pharmacokinetic and pharmacodynamic studies.
Most of the drugs in multi-component dosage forms can be analyzed by HPLC method because of the several advantages like rapidity, specificity, accuracy, precision and ease of automation in this method. HPLC method eliminates tedious extraction and isolation procedures. Some of the advantages are:
- Speed (analysis can be accomplished in 20 minutes or less),
- Greater sensitivity (various detectors can be employed),
- Improved resolution (wide variety of stationary phases),
- Reusable columns (expensive columns but can be used for many analysis),
- Ideal for the substances of low volatility,
- Easy sample recovery, handling and maintenance,
- Instrumentation tends itself to automation and quantitation (less time and less labour),
- Precise and reproducible,
- Calculations are done by integrator itself.
1.4.2 Mode of Separation
There are different modes of separation in HPLC. They are normal phase mode, reversed phase mode, reverse phase ion pair chromatography, affinity chromatography. Normal phase mode, the stationary phase is polar and the mobile phase is nonpolar in nature. In this technique, nonpolar compounds travel faster and are eluted first. Reversed phase mode is the most popular mode for analytical and preparative separations of compound of interest in chemical, biological, pharmaceutical, food and biomedical sciences. The polar compound gets eluted first in this mode and nonpolar compounds are retained for longer time. As most of the drugs and pharmaceuticals are polar in nature, they are not retained for longer times and hence elute faster.
1.5 Method Development in HPLC
A typical method development scheme has the following steps:
1. Choose the mode
2. Choose the column and column packing dimensions
3. Choose the stationary phase chemistry
4. Choose the mobile phase solvents
5. If the mode requires it, adjust the mobile phase pH
6. Run some initial isocratic or gradient experiments to define boundary conditions
7. Optimize the experimental conditions
1.6 Method Optimization
The aim of this step is to achieve adequate selectivity (peak spacing). The mobile phase and stationary phase compositions need to be taken into account. To minimize the number of trial chromatograms involved, only the parameters that are likely to have a significant effect on selectivity in the optimization must be examined. For the final method optimization following parameters should be considered.
- Shorter analysis time
- Better Resolution
- Better selectivity
- Better Sensitivity
After method optimization, system parameter optimization also considered. This is used to find the desired balance between resolution and analysis time after satisfactory selectivity has been achieved. The parameters involved include column dimensions, column-packing particle size and flow rate. These parameters may be changed without affecting capacity factors or selectivity.
2. AIM AND OBJECTIVE
To develop an HPLC method for simultaneous estimation of Montelukast and Fexofenadine by HPLC.
- Literature assessment showed that various analytical methods has been reported for the estimation of Montelukast and Fexofenadine individually and with combination also but combination does not fulfill regulatory requirement and with other drugs combination by HPLC in pharmaceutical dosage form.
3. WORK PLAN:
* Method Development:
· Study of physicochemical parameters.
- Solubility
- Maximum Absorbance
- Molecular Weight
· Selection of Suitable Stationary Phase.
- Normal Phase
- Reversed Phase
· Selection of Suitable Mobile Phase Depending Upon Polarity
· Selection of Appropriate Columns.
· Selection of Proper Temperature.
· Selection of Proper Flow Rate.
· Selection of Detection Wavelength.
· Selection of Sampling Preparation.
4. DRUG PROFILE
4.1 Montelukast:
Table 1: Properties of Montelukast
|
Chemical name |
(S,E)-2-(1-((1-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propylthio)methyl)cyclopropyl)acetic acid |
|
Mol. Formula |
C35H36ClNO3S |
|
Mol. Wt |
608.18 g/mol |
|
Physical appearance |
White or off-white powder, hygroscopic |
|
Melting Point |
275.9oC |
|
Solubility |
Freely soluble in ethanol, methanol and water and practically insoluble in acetonitrile. |
|
Category |
Leukotriene Blocker |
|
Wavelength |
253 nm |
4.1.1 Mechanism of Action:
Montelukast is a CysLT1 antagonist; it blocks the action of leukotriene D4 (and secondary ligands LTC4 and LTE4) on the cysteinyl leukotriene receptor CysLT1 in the lungs and bronchial tubes by binding to it. This reduces the bronchoconstriction otherwise caused by the leukotriene and results in less inflammation.
4.1.2 Adverse Effects:
Adverse effects include gastrointestinal disturbances, headaches, hypersensitivity reactions, sleep disorders, and increased bleeding tendency, in addition to other generic adverse reactions. Its use is associated with a higher incidence of Churg–Strauss syndrome. Drowsiness is also a common side effect.
4.1.3 Uses:
Asthma is a common condition caused by inflammation in the airways. It is not known why the inflammation occurs. The inflammation irritates the muscles around the airways, and causes them to constrict. This causes your airways to narrow. It is then more difficult for air to get in and out of your lungs. This causes the typical symptoms of wheeze, chest tightness, and shortness of breath.
Asthma symptoms flare up from time to time and there may be no apparent reason why. However, some people find that symptoms are made worse by triggers such as exercise, fumes, and pollen. These things cause your body to produce chemical substances called leukotrienes, which cause inflammation. Montelukast helps control the symptoms of asthma by blocking the effects of these leukotrienes.
4.2 Fexofinadine:
Table 2: Properties of Fexofinadine
|
Chemical name |
2-(4-{1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperidin-1-yl]butyl}phenyl)-2-methylpropanoic acid |
|
Mol. Formula |
C32H39NO4•HCl |
|
Mol. Wt |
501.65 g/mol |
|
Physical appearance |
White, odorless powder |
|
Melting Point |
142.5 oC |
|
Solubility |
Freely soluble in methanol and ethanol, slightly soluble in chloroform |
|
Category |
Antihistamine with selective peripheral H 1-receptor antagonist activity |
|
Wavelength |
290 nm |
4.2.1 Mechanism of Action:
Like other H1-blockers, Fexofenadine competes with free histamine for binding at H1-receptors in the GI tract, large blood vessels, and bronchial smooth muscle. This block the action of endogenous histamine, which subsequently leads to temporary relief of the negative symptoms (eg. nasal congestion, watery eyes) brought on by histamine.
4.2.3 Adverse Effects:
Anaphylaxis and hypersensitivity reactions (chest tightness; feeling of warmth redness of the face, neck, arms and occasionally, upper chest; large, hive-like swelling on face, eyelids, lips, tongue, throat, hands, legs, feet, sex organs; shortness of breath, difficult or labored breathing).
4.2.4 Uses:
Fexofenadine hydrochloride (Allegra) is an antihistamine drug used in the treatment of hayfever and similar allergy symptoms. Fexofenadine, like other second and third-generation antihistamines, does not readily pass through the blood-brain barrier, and so causes less drowsiness than first-generation histamine-receptor antagonists.
5. EXPERIMENTAL WORK
5.1 Solubility Study
The solubility of Montelukast and Fexofenadine were practically determined as per IP 2007: Solubility was determined by taking 100 mg of Montelukast and Fexofenadine in 50 mL volumetric flasks, adding required quantity of solvent at room temperature and shaken for few minutes. Solubility data for each study was observed and recorded in Table 3.
Table 3: Solubility Data of Montelukast and Fexofenadine
|
Solvent |
Solubility |
|
|
Montelukast (Observed) |
Fexofenadine (Observed) |
|
|
Water |
Soluble |
Soluble |
|
Methanol |
Freely soluble |
Freely soluble |
|
Acetonitrile |
Freely soluble |
Freely soluble |
Chromatographic Separation can be demonstrated by a sufficient separation of the substances present. For the assay, appropriate separation means an adequate resolution between the peak of interest and other peaks (e.g., impurities, placebo or matrix components). So, several trials for the development purpose were taken for the simultaneous estimation of Montelukast and Fexofenadine.
Trial 1:
* Mobile phase- Buffer : ACN –(80:20)
* Buffer pH – 4.9
*-Conditions:
- Column – Water Spherisob ODS 1 (250*4.6) 5 um.
- Injection volume – 50 ul.
- Flow - 1.2 ml/min
* Observation –Montelukast peak shape was not good.
* Conclusion – Modified M.P. and reduced injection volume.
Trial 2:
* Mobile Phase – Buffer : MeOH –(70:30)
* Buffer pH – 4.3
* Conditions:
- Column – Water Spherisob ODS 1 (150*4.6) 5 um.
- Injection volume – 50 ul
- Flow – 1.2 ml/min
Observation – Montelukast and Fexofenadine peak shape was not found proper and was late eluting.
Trial 3:
* Mobile Phase – Buffer : ACN (85:15)
* Buffer pH – 4.3
* Conditions:
- Column – Kromasil 100 C18 (250*4.6 mm) 5um.
- Injection Volume - 50 ul
- Flow – 1.0 ml/min.
* Observation – Response of Montelukast and Fexofenadine peak was found to be not proper and hump was found.
Trial 4:
* Mobile Phase – Buffer : MeOH (50:50)
* Buffer pH – 3.9
* Conditions:
- Column – BDS hypersil C18 (250 * 4.6 mm) 5um
- Injection Volume - 50 ul
- Flow – 1.0 ml/min
* Observation – Response of Montelukast and Fexofenadine peak was found to be not proper.
Trial 5:
* Mobile phase - Buffer : MeOH (20:80)
* Buffer pH – 4.0
* Conditions:
- Column – BDS hypersil C18 (250 * 4.6 mm) 5um
- Injection Volume - 20 ul
- Flow – 1.0 ml/min
* Observation – Peak shape of Fexofenadine was not proper.
Trial 6:
* Mobile phase - Buffer : MeOH (30:70)
* Buffer pH – 4.0
* Conditions:
- Column – BDS hypersil C18 (250 * 4.6 mm) 5um
- Injection Volume - 20 ul
- Flow – 1.0 ml/min
* Observation - Peak shape of Montelukast and Fexofenadine was solved and was found Proper
* So, in Trial 6 peak shape of Montelukast and Fexofenadine was resolved against several trials and was found to be proper.
* So, Trial 6 was taken into consideration and further Analytical Method Development for the Simultaneous Estimation of Montelukast and Fexofenadine by HPLC was demonstrated.
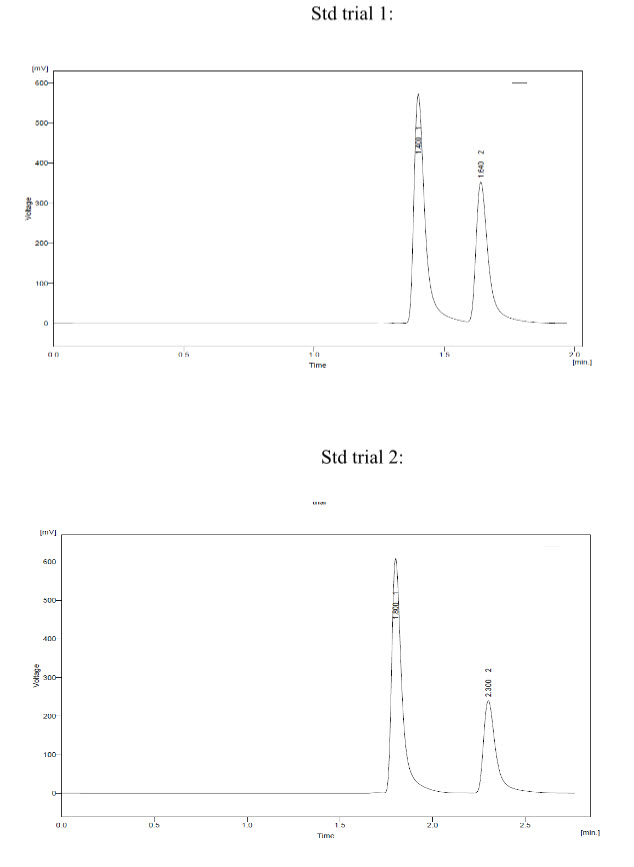
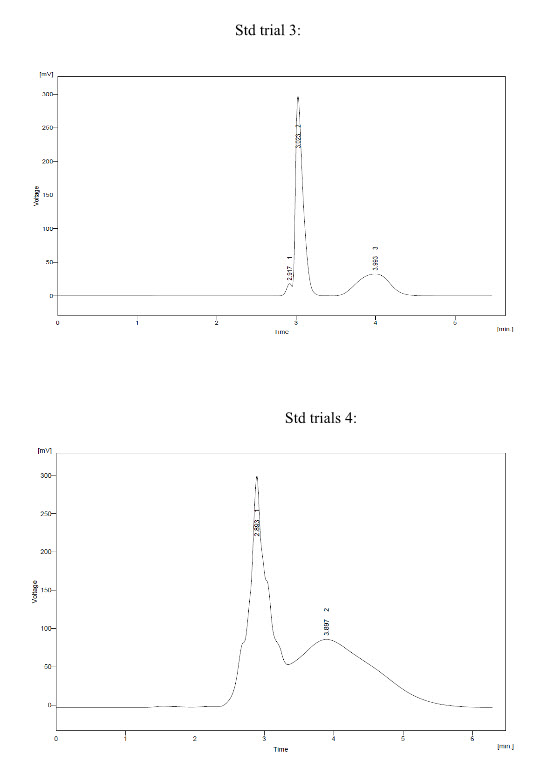
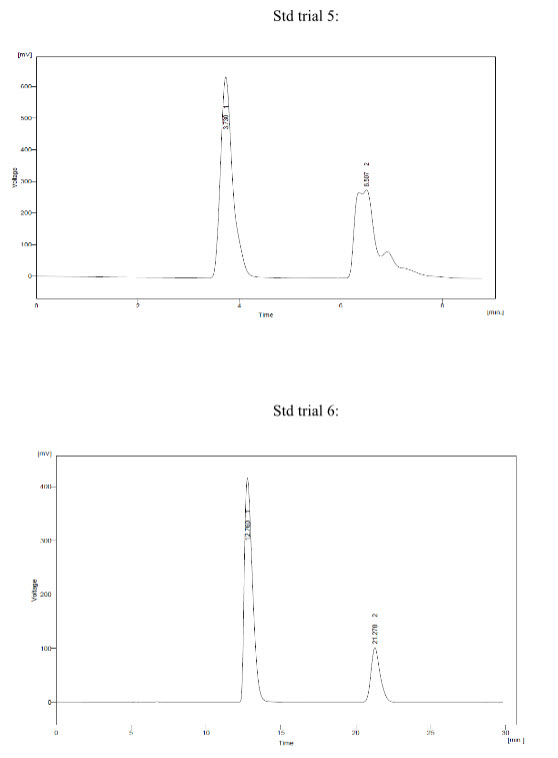
5.2 Estimation by Developed HPLC Method
• Buffer Preparation pH 4.0:
Dissolve 1gm of Potassium dihydrogen phosphate in 1000 ml in Milli-Q water, stir well and adjust the pH to 4.0 with diluted Orthophosphoric acid and Filtered.
• Preparation of Mobile Phase:
Prepare Sonicate and degassed mixture of Buffer pH 4.0 and MeOH in the ration of 30:70.
• Diluent:
Prepare Sonicate and degassed mixture of Buffer pH 4.0 and MeOH in the ration of 30:70.
• Preparation of Standard Stock Solution A of Montelukast:
Transfer an accurately weight quantity of about 100 mg of Montelukast working standard to a 100 ml volumetric flask, sonicate to dissolve and diluent to volume and mix well.
• Preparation of Standard Stock Solution B of Fexofinadine:
Transfer an accurately weight quantity of about 120 mg of Fexofinadine working standard to a 100 ml volumetric flask, sonicate to dissolve and diluent to volume and mix well.
• Preparation of Final Standard Solution of Montelukast and Fexofinadine:
Transfer an accurately weight quantity of about 12 mg of Fexofinadine and 1 mg of Montelukast stock solution mix in the 25 ml with mobile phase, sonicate to dissolve andd mix well
• Sample Prepration for Assay:
1/10 tablet powder was dissolved in 25 ml mobile phase.
Optimized Chromatographic Conditions for Montelukast and Fexofinadine:
• Column: BDS hypersil C18 (250 * 4.6 mm) 5um
• Detector: 230 nm
• Injection Volume: 20 µL
• Flow Rate: 1.0 mL/min
• Temperature: 40 ºC
• Run Time: 30 minute
• Mobile Phase: Buffer: MeOH (30:70)
6. RESULT AND DISCUSSION
- The main objective of this work was to develop and validate HPLC method for simultaneous estimation of Montelukast and Fexofenadine in P’ceutical dosage form by HPLC. So, Method development of drug was done and after observing several trilas in different Chromatographic condition the peak issue that is late elution, tailing, frounting, splitting, less resolution etc. was resolved and was Observed proper. . The method has provided adequate separation for Montelukast and Fexofenadine. Separation was obtained by using a BDS Hypersil C18 (250 * 4.6 mm) 5um at 40 °C temperature and using a mobile phase Buffer (pH 4): MeOH in the ratio of (30:70) at a flow rate 1.0 mL/min and wavelength for detection was 230 nm. So, the retention time and theoretical plates was found to be:
|
Sr. No. |
Parameters (n= 5) |
Montelukast |
Fexofenadine |
|
1 |
Retention Time (min) |
21.270 |
12.760 |
|
2 |
Area |
15642.838 |
4266.303 |
- So, in Trial 6 peak shape of Montelukast and Fexofenadine was resolved against several trials and was found to be proper.
- So, Trial 6 was taken into consideration and further Analytical Method Development for the Simultaneous Estimation of Montelukast and Fexofenadine by HPLC was demonstrated.
- The observed RT of peak and run time is sufficient and all conditions of this method are acceptable, so, method was finalized.
7. REFERENCES
1.Mendham J, Denny R.C., Barnes J.D. and Thomas M.J.K., “Vogel’s Textbook of Quantitative Chemical Analysis”, 6th ed., India: Dorling Kindersley (India) Pvt. Ltd. 2008, pp. 5-11, 244-271.
2.Skoog D.A. and West D.M., “Principles of Instrumental Analysis. 2nd ed. Philadelphia: Sauners college, 1980, pp.1-4, 690-699.
3.Chatwal G.R. and Anand, “Instrumetnal Methods of Chemical Analysis”, Mumbai: Himalaya Publishing House, 2000, pp. 180-98.
4.Brown P. and Deantonis K. “HPLC Handbook of Instrumental Techniques for Analytical Chemistry”, ed 1997, New Jersey: Prentice Hall. Inc., pp. 2-7.147-157.
5.Sharma B.K, “Instrumental Method of Chemical Analysis”, 25th ed. New Delhi: GOEL Publishing House, 2006, pp. 678-682, 2.625-2.639.
6.Okafo G.N. and Roberts, J.K. (2008), “Development of achiral separation method in Pharmaceutical analysis”, In: Lee, D.C. and Webb, M.L. ed. Pharmaceutical analysis. New Delhi: Wiley India Pvt. Ltd. Blackwell Publishing, 2008, pp. 90, 31-48.
7.Munson J.B., “Pharmaceutical Analysis Modern Methods”, Mumbai: International Medical Book Distributors, 2001, pp. 378.
8.Willard H.H., Merritt L.L., Dean J.A. and Settle F.A., “Instrumental Method of Analysis”, 7th ed., New Delhi: CBS Publishers and Distributors, 2001, pp. 170.
9.Schirmer R.E., “Modern Methods of Pharmaceutical Analysis”, 2nd ed., Floride: CRC Press, 2000, pp. 67-72, 239-384.
10.Sabir G.A., “HPLC Method Development and Validation for Pharmaceutical Analysis” May 2004, pp.164.
11.Sethi P.D., “HPLC Quantitative Analysis of Pharmaceutical Formulation”, 1sted.,: CBS Publishers and Distributors New Delhi,2001, pp. 1-20.
12.Ahuja S. and Dong M.W., “Handbook of Modern Pharmaceutical Analysis by HPLC”, 1st ed.: Elseveir India Pvt. Ltd New Delhi, 2005, pp. 24-30.
13.Ahuja S. and Scypinski S., “Handbook of Modern Pharmaceutical Analysis”, London: Academic press, 2001, pp.349-373.
14.ICH, Q2A “Validation of Analytical Procedure: Text and Methodology”, International Conference on Harmonization, Geneva, Switzerland, 2005.
15.Synder R, Joseph J, Kirkland and Joeph L G, “Practical HPLC Method Development” 2nd Edition, IIyod.
16.Dong M.W., “Modern HPLC for practicing scientists”, Jhon wiley & sons. Inc New Jersey, 2006, pp. 1- 46.
17.Kealey D. and Haines P.J., “Instant Notes Analytical Chemistry”, 1st ed. Bios scientific publisher Ltd. UK, pp. 109-130, 155-173.
18.George L., Schmuff N., “HPLC Methods for Pharmaceutical Analysis”, John Wiley and Sons, Inc. New York, 1996, pp. 433-434.
19.Snyder L.R., Kirkland J.J. and Glaich, J.L., “Practical HPLC method development” 2nd ed., pp. 112-114.
20.Meyers R.A., “Encyclopedia of Analytical Chemistry: Application, Theory and Instrumentation”, New Jersey, Wiley, Oct.2000.
21.Watson D.G., “Pharmaceutical Analysis: A textbook for pharmacy students and pharmaceutical chemists” 2nd ed. London: Churchill Livingstone, 2000, pp.195-205. 236-276.
22.Prichard E., “Analytical Measurement Terminology: Handbook of terms used in Quality Assurance of Analytical measurement” UK: Royal Society of Chemistry, pp. 9-27.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









