ABOUT AUTHORS:
Bhatt Preeti *, Gnanaranjan. G , kothiyal Preeti
Department of pharmaceutics,
Shri Guru Ram Rai Institute Of Pharmacy,
Dehradun-248001, India
*preetibhatt03@gmail.com
ABSTRACT
The motto of writing this review on additives in pharmaceutical gel was to compile the recent literature with special focus on rational approach to topical formulation and basic components used in gel for topical drug delivery systems. Topical drug delivery systems involve the introduction of a drug to the surface of the body, in a formulation which can be absorbed. Topical gels are intended for skin application or to certain mucosal surfaces for local action or percutaneous penetration of medicament or for their emollient or protective action as they show better potential as a vehicle to administered drug topically in comparison to ointment. Additives are inactive ingredients used for structuring dosage form. Selection of topical vehicles depends on various dermatological factors and pharmaceutical factors. Dermatological factors are absorption penetration, skin condition, compatibility, emollient properties. Pharmaceutical factors are stability, solvent properties, emulsifying property. The choice of suitable exepient for a formulation development is made on the basis of the drug delivery requirements and the particular need to impart sufficient emolliency or other quasi-medicinal qualities in the formulation.
Reference Id: PHARMATUTOR-ART-1988
INTRODUCTION
Skin
Anatomy and physiology
The skin acts as a boundary which separates the external environment from the internal organs. The skin provides physical protection of internal organs and acts as a sensory organ. It controls body temperature and water loss, and functions as a regulatory barrier which controls the movement of substances into and out of the body. The skin has gained increasing favour as a target site for drug delivery as it avoids problems associated with oral drug administration, namely pH and to some extent, enzyme driven drug degradation and hepatic first-pass metabolism.
There are numerous diseases which affect different regions of the skin. Any drug used will be required to reach the site of the disease in order to exert its pharmacological activity. Unless it is for a local effect on the surface only, the drug must either pass through the stratum corneum(SC) or go through hair follicles or sweat glands to reach its target site. Once in the skin, a lipid-soluble drug will tend to accumulate in lipophilic regions while more water-soluble drugs will tend to enter the blood capillaries and are removed from the skin [1].
Skin is composed of three primary layers-
A. Epidermis
B. Dermis
C. Hypodermis
Epidermis
An important function of the epidermis is the generation of the stratum corneum. The epidermis is avascular and is sustained by nutrients it receives by diffusion from the underlying dermal capillaries through the basement membrane. The epidermal layer forms as a result of the death of basal cells via a specialised differentiative process. Epidermis further divided into the following2 :-
1. Stratum corneum
2. Stratum lucidum
3. Stratum granulosum
4. Stratum spinosum
5. Stratum Basale
Cells are formed through mitosis at the basale layer. The cytoplasm is released and the protein keratin is inserted. They eventually reach the corneum and slough off (Desquamation). This process is called keratinization and takes place within about 30 days. This keratinized layer of skin is responsible for keeping water in the body and keeping the harmful chemicals and pathogens out.
GELS
A gel is an intermediate state of matter possessing property of a solid and a liquid, termed as viscoelasticity. It is a two-component, cross linked three-dimensional network consisting of structural materials interspersed by an adequate but proportionally large amount of liquid to form an infinite rigid network structure which immobilizes the liquid continuous phase within4. The structural materials that form the gel network can be composed of inorganic particles or organic macromolecules, primarily polymers 5.
Properties of gels
Gels should posses the following properties7 :-
1. Ideally, the gelling agent for pharmaceutical or cosmetic use should be inert, safe, and should not react with other formulation components.
2. It should possess suitable anti-microbial to prevent from microbial attack.
3. The gelling agent included in the preparation should produce a reasonable solid-like nature during storage that can be easily broken when subjected to shear forces generated by shaking the bottle, squeezing the tube, or during topical application.
4. The ophthalmic gel should be sterile.
5. The topical gel should not be tacky.
ADDITIVES IN GEL
Polymers are used to give the structural network, which is essential for the preparation of gels. Gel forming polymers are classified in table no.1.
Gelling agent
Gelling agent are hydrocolloids substance which gives thixotrophic consistency to the gel. Gelling agents are organic in nature. Gelling agents are also known as solidifiers or stabilizer and thickening agent. Gelling agents are more soluble in cold water than hot water. Gelling agents like methylcellulose and polaxamers have better solubility in cold water while bentonite, gelatin and sodium carboxymethylcellulose are more water soluble in hot water. Gelling agents are used in concentration of 0.5 up to 10% depending on the agent most gelling agents require 24-48 hours to completely hydrate and reach maximum viscosity and clarity. It is easier to add the active drug before the gel is formed if the drug does not interfere with the gel formation. The viscosity of the gelling agents in the gelling layer be within range of about 1000 cps to about 100,000 cps. Various hydrocolloids used as gelling agents are:
1. Natural polymer
a. Proteins
i) Gelatin:-
- Gelatin is a common natural polymer ( water soluble polymer) or protein which is normally produced by denaturing collagen [8].
- In addition, gelatin can be easy to manipulate due to its isoelectric point that allows it to change from negative to positive charge in an appropriate physiological environment or during the fabrication, a property that has found it being very attractive to many pharmaceutical researchers[10] .
- Gelatin is one of the natural polymers used as support material for gene delivery, cell culture, and more recently tissue engineering. Gelatin-based systems have the ability to control release of bioactive agents such as drugs, protein, and dual growth factors[11, 9, 12] .
- However, some setbacks have been identified, and they are said be associated with the use of gelatin-based systems in pharmaceutical applications. These setbacks include poor mechanical strength and ineffectiveness in the management of infected sites[13].
ii) Collagen:-
Collagen is a major natural protein component in mammals that is fabricated from glycine-proline-(hydroxy) proline repeats to form a triple helix molecular structure[14]. Till now nineteen types of collagen molecules have been isolated, characterized, and reported in both medical and pharmaceutical applications[13-15]. collagen gels are one of the first natural polymers to be used as a promising matrix for drug delivery and tissue engineering[16].
b. Polysaccharides
Natural polysaccharides are extensively used for the development of solid dosage forms. These polymers of monosaccharide’s (sugars) are inexpensive and available in a variety of structures with a variety of properties. They are highly stable, safe, non-toxic, and hydrophilic and gel forming in nature. Pectin’s, starch and amylase are a few polysaccharides commonly used in controlled release dosage forms. Non-starch, linear polysaccharides remain intact in the physiological environment of the stomach and the small intestine, but are degraded by the bacterial inhabit-ants of the human colon which make them potentially useful in targeted delivery systems to the colon[17].
i) Pectin:-
Pectins are non-starch, linear polysaccharides extracted from the plant cell walls[18]. In the food industry, folic acid incorporated microcapsules were prepared using alginate and combinations of alginate and pectin polymers so as to improve stability of folic acid. The blended alginate and pectin polymer matrix increased the folic acid encapsulation efficiency and reduced leakage from the capsules as compared to those made with alginate alone, they showed higher folic acid retention after freeze drying and storage[19].
Tragacanth
Natural complex polysaccharide variable in its rheological property and its microbiological quality. It is the sap of legumes of several species of genus Astralagus plant. It is viscous, odourless, and tasteless of which 5% concentration is used in medicated gels. It must be pre-wetted with ethanol or glycerin before dispersion in water. As amucilage or paste it used as topical treatment for burns. It is acidic in nature and has high molecular weight 840, 0000 and yields glucoronic acid and arabinose when hydrolysed. It acts as demulscent and suspending agent. Complex glucoarabinose polysaccharides isolated from a related asian species, which stimulate the production of T-cells and antibody producing plasma cells. The different types of flakes of tragacanth gum are shown in figure no.3.
2.Semi-synthetic polymers
a. Cellulose derivatives
Cellulose is the most abundant naturally occurring biopolymer [20,21]. It consists of long chains of anhydro-D-glucopyranose units (AGU) with each cellulose molecule having three hydroxyl groups per AGU, with the exception of the terminal ends.
Cellulose is insoluble in water and most common solvents [21] ; In spite of its poor solubility characteristics, cellulose is used in a wide range of applications including composites, netting, upholstery, coatings, packing, paper, etc.
Chemical modification of cellulose is performed to improve process ability and to produce cellulose derivatives (cellulosics) which can be tailored for specific industrial applications [22]. Large scale commercial cellulose ethers include carboxymethyl cellulose (CMC), methyl cellulose (MC), hydroxyethylcellulose (HEC), hydroxypropyl methyl cellulose (HPMC), hydroxylpropyl cellulose(HFC), ethyl hydroxyethyl cellulose (EHEC), and methyl hydroxyethyl cellulose(MHEC).
i).Hydroxyethyl cellulose :- It is ether like methyl cellulose where the substituent group is hydroxyl ethyl and It forms an occlusive dressing when lightly applied to the skin and allow to dry.
ii). Methyl cellulose:-It is non-ionic and stable over a wide spectrum of pH. It is soluble in cold water but insoluble in hot water. Compatible with water, alcohol (70%), and propylene glycol (50%). Marketed methyl cellulose is Methocel HG and Methocel MC.
iii). Carboxymethyl cellulose :- It isGenerally used as the sodium salt known as carmellose sodium. It contains carboxy methyl group (- CH2 COOH) and makes thicker gels but less tolerance than hydroxyl propyl methylcellulose. Maximum stability at pH 7-9.
iv). Hydroxypropyl cellulose :- It Makes thinner gels with high tolerance for added drugs and saltsHydrates and swells in water or hydroalcoholic solution.
v). Hydroxypropyl methyl cellulose :-It Makes thicker gels but lower tolerance for positively charge ions.Disperse in cool water. It is a Good gelling agent for time released formulation.
3. Synthetic polymers
a. Carbomer
Carbomer polymers are polymers of acrylic acid cross-linked with polyalkenyl ethers or divinyl glycol. They are produced from primary polymer particles of about 0.2 to 6.0 micron average diameter. The flocculated agglomerates cannot be broken into the ultimate particles when produced. Each particle can be viewed as a network structure of polymer chains interconnected via cross-linking23.
Carbopol polymers are offered as fluffy, white, dry powders (100% effective).
The carboxyl groups provided by the acrylic acid backbone of the polymer are
responsible for many of the product benefits. Carbopol polymers have an average
equivalent weight of 76 per carboxyl group24. The general structure
can be illustrated with fig. No.4.
Poloxamers (Pluronics)
- Poloxamers are copolymer of polyoxy ethylene and polyoxy propylene.
- They form thermoreversible gels in concentration ranging from 15 to 50%.
- Poloxamers are white, waxy granules that form clear liquid when dispersed in cold water.
- “PLO” gel formed by combining pluronic F-127 with lecithin an isopropyl palmitate.
- Pluronic F-127 is a grade of poloxamer.
Polyvinyalcohol
It is less soluble in cold water. It used at 2.5% concentration to prepare gel. Borax often adds in PVA solution. It is formed from ploymerised vinyl acetate.
4. Inorganic substances
Bentonite
It is an absorbent aluminium phyllosilicate, essentially impure clay consisting mostly of montmorillonite28 . Montmorillonite is superfine flaky aluminosilicate, where due to lattice nestehiometrical cations exchange surplusnegative charge appears and compensates cation-exchange located in interlayer area. High waterproofing is conditioned on this. In case of water tempering, water penetrates in interlayer area of montmorillonite, hydrates it's surface and cation-exchange, so that provides swelling of the mineral. During further water dilution bentonite forms firm viscous suspension with thixotropic properties. Montmorillonite has high cation-exchange and adsorption properties.
Owing to this properties bentonite has found wide application as viscous gelling agent and filtration diminishing agent in drilling muds production for well-boring, as binding agent in foundry sands and iron ore pellets and also as hydraulic and adsorption material.
Humectant and cosolvants in gel
Humectant is a substance that absorbs or helps another substance retain moisture, as glycerol. It is a hygroscopic substance or often a molecule with several hydrophilic groups, most often hydroxyl groups, but amines and carboxyl groups, sometimes esterifies, can be encountered as well.
Some of the examples are as follows:-
- Glycerine, propylene glvcol (E 1520) and giyceryl triacetate (EI5I8).
- Others can be polyols like sorbitol (E420), xylitol and maltitoi (E965).
- Or polymeric polyols like polvdextrose (El 200)
- Or natural extracts like quillaia (E999), or lactic acid or urea.
Lithium Chloride is an excellent humectant.
Stabilizers
Bases and medicaments sensitive to heavy metals are sometimes protected by chelating agent, such as E.D.T.A.(Ethlylene diamine tetra acetic acid).
SUMMARY AND CONCLUSION
Additives are inactive ingredients in dosages form. Additives are non drug component for structuring dosage form. Selection of topical vehicles depends on various dermatological factors and pharmaceutical factors. Natural polymers have proven their potential for the development of new, advanced and efficient drug delivery system. Gel is most widely used semisolid topical dosage form using various gelling agents such as tragacanth, pectin, gelatin, cellulose derivatives, carbopols, polaxamers, alginates in gel formation. Humectant is a hygroscopic substance. It is used in almost all topical dosages forms except topical powder. Its function is retaining moisture by absorb water from air. Preservatives act as stabilizer in topical dosages forms. Paraben, benzoic acid, phenol, benzalkonium chloride, actamer is used as preservative in topical dosages forms.
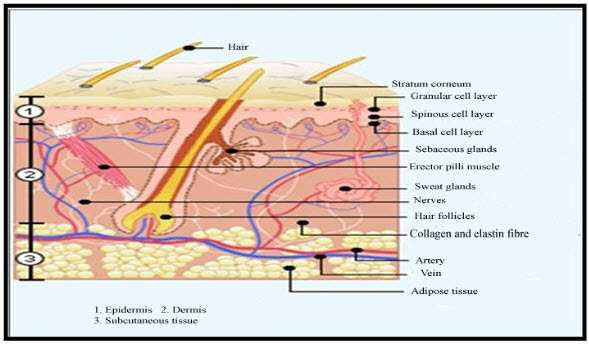
Figure no.1: Transverse Section of Human Skin [3]

Figure no.2: gelatin powder
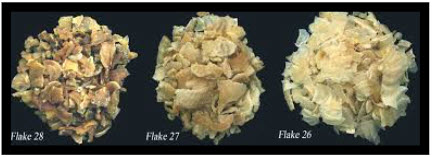
figure no.3: tragacanth gum flakes
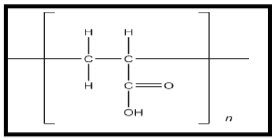
Figure no.4: general structure of carbopol
Table no.1: Gel forming polymers7
|
S.NO. |
POLYMER |
EXAMPLE |
|
1. |
Natural polymer a. Proteins b. Polysaccharides |
a.i. Gelatin ii. Collagen b.i. Alginic acid ii. Agar iii. Tragacanth iv.Sodium or Potassium carrageenan v. Pectin vi. Gellum Gum vii. Xanthin viii. Cassia tora ix. Guar Gum |
|
2. |
2. Semisynthetic polymers a. Cellulose derivatives |
i. Hydroxyethyl cellulose ii. Methylcellulose iii. Hydroxypropyl methyl cellulose iv. Hydroxypropyl cellulose v. Carboxymethyl cellulose |
|
3. |
3. Synthetic polymers a. Carbomer
b. Poloxamer c. Polyvinyl alcohol d. Polyacrylamide e. Polyethylene and its co-polymers |
a.i. Carbopol -941 ii. Carbopol -940 iii. Carbopol -934 |
|
4. |
4. Inorganic substances |
a. Bentonite b. Aluminium hydroxide |
|
5. |
5. Surfactants |
a. Brij-96 b. Cetostearyl alcohol |
Table No.2: Physical and Chemical Properties of Carbopol 25
|
Appearance |
Fluffy, white, mildly acidic polymer |
|
Bulk Density |
Approximately 208 kg/m3 (13lbs.ft3) * |
|
Specific gravity |
1.41 |
|
Moisture content |
2.0% maximum |
|
Equilibrium moisture content |
8-10% (at 50% relative humidity) |
|
PKa |
6.0 ± 0.5 |
|
pH of 1.0% water dispersion |
2.5 - 3.0 |
|
pH of 0.5% water dispersion |
2.7 - 3.5 |
|
Equivalent weight |
76 ± 4 |
|
Ash content |
0.009 ppm (average) ** |
|
Glass transition temperature |
100-105C (212-221F) |
* Polymers produced in co solvent (a cyclohexane / ethyl acetate mixture) have a bulk density of 176 kg/m3 (11 lbs/ft3).
* * Polymers produced in ethyl acetate have an ash content (as potassium sulfate) of 1-3% on average.
Table No.3: Viscosity range of different Carbopol Polymers26,27
|
S.NO |
POLYMER NAME |
VISCOSITY* |
PROPERTIES |
|
1. |
Carbopol®910 |
3,000-7,000 |
Effective in low concentrations and wi ll provide a low viscosi ty formulation. |
|
2. |
Carbopol®934 |
30,500-39,400 |
Effective in thick formulations such as emulsions, suspensions, sustain release formulations, transdermals, and topicals forms clear gels with water. |
|
3. |
Carbopol® 934P |
29,400-39,400 |
Same properties as 934, but intended for pharmaceutical formulation purified product. |
|
4. |
Carbopol® 940 |
40,000-60,000 |
Effective in thick formulations, very good clarity in water or hydro alcoholic topical gels. Form clear gels with hydro alcoholic systems. |
|
5. |
Carbopol® 941 |
4,000-11,000 |
Produces low vi scosi ty gels, very good clarity |
* Brookfield RVT Viscosity, cP 0.5 wt % mucilage at pH 7.5, 20 rpm at 25oC
REFRENCES
[1] Johnson ME, Blankschein D, Linger R. Evaluation of solute permeation through the stratum corneum lateral bilayer diffusion as the primary transport mechanism. J Pharm Sci 1997; 86 (10):1162-1172.
[2] Elias J. The Microscopic Structure of the Epidermis and Its Derivatives. In: Percutaneous Absorption, 2nd Edition, Eds Bronaugh R L, Maibach H I, New York: Marcel Dekker, Inc; 1989. 3-12.
[3] Skin biology and structure. MIMS 2007.
[4] Larson RG: The structure and rheology of complex fluids. Oxford University Press, New York; 1999.
[5] Alvarez-Lorenzo C and Concheiro A: Effects of surfactants on gel behavior. Design implications for drug delivery systems. Am. J. Drug Deliv. 2001; 1 (2): 77-101.
[6] Carter SJ: Disperse system In: Cooper and Gunn’s Tutorial Pharmacy. 6th ed. New Delhi: CBS Publishers and Distributors; 2000: 68-72.
[7] Goyal S, Sharma P, Ramchandani U, Shrivastava SK and Dubey PK: Novel anti-inflammatory topical gels. International Journal of Pharmaceutical and Biological Archives. 2011; 2(4): 1087-1094.
[8] S. Hao, L. Li, X. Yang et al., ?The characteristics of gelatin extracted from sturgeon (Acipenser baeri) skin using various pretreatments,? Food Chemistry, vol. 115, no. 1, pp. 124–128, 2009.
[9]. A. Narita, M. Takahara, T. Ogino, S. Fukushima, Y. Kimura, and Y. Tabata, ?Effect of gelatin hydrogel incorporating fibroblast growth factor 2 on human meniscal cells in an organ culture model,? The Knee, vol. 16, no. 4, pp. 285–289, 2009.
[10]. S. Young, M. Wong, Y. Tabata, and A. G. Mikos, ?Gelatin as a delivery vehicle for the controlled release of bioactive molecules,? Journal of Controlled Release, vol. 109, no. 1–3, pp. 256–274, 2005.
[11]. T. A. Holland, Y. Tabata, and A. G. Mikos, ?Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering,? Journal of Controlled Release, vol. 101, no. 1–3, pp. 111–125, 2005.
[12]. K. Ofokansi, G. Winter, G. Fricker, and C. Coester, ?Matrix-loaded biodegradable gelatin nanoparticles as new approach to improve drug loading and delivery,? European Journal of Pharmaceutics and Biopharmaceutics, vol. 76, pp. 1–9, 2010.
[13]. R. Parenteau-Bareil, R. Gauvin, and F. Berthod, ?Collagen-based biomaterials for tissue engineering applications,? Materials, vol. 3, no. 3, pp. 1863–1887, 2010.
[14]. C. Kojima, S. Tsumura, A. Harada, and K. Kono, ?A collagen-mimic dendrimer capable of controlled release,? Journal of the American Chemical Society, vol. 131, no. 17, pp. 6052–6053, 2009.
[15]. C. Holladay, M. Keeney, U. Greiser, M. Murphy, T. O'Brien, and A. Pandit, ?A matrix reservoir for improved control of non-viral gene delivery,? Journal of Controlled Release, vol. 136, no. 3, pp. 220–225, 2009.
[16]. A. L. Weiner, S. S. Carpenter-Green, and E. C. Soehngen, ?Liposome-collagen gel matrix: a novel sustained drug delivery system,? Journal of Pharmaceutical Sciences, vol. 74, no. 9, pp. 922–925, 1985.
[17]. Sinha V.R., Rachna K.. Polysaccharides in colon specific drug delivery. Int. J. Pharm. 2001; 224:19-38.
[18]. Sahasathian T., Kerdcholpetch T., Chanweroch A., Praphairaksit N., et al. Sustain release of amoxicillin fro chitosan tablet. Archives of pharma res. 2007; 40:526-531.
[19]. Madziva H., Kailasapathy K., Phillips M.. Alginate-pectin microcapsules as a potential for folic acid delivery in foods. J Microencap. 2005; 22:343–51.
[20]. Hinterstoisser B., Salmen L.: Application of dynamic 2D FTIR to cellulose. Vibrational Spectroscopy, 22, 111–118 (2000).
[21]. Bochek A. M.: Effect of hydrogen bonding on cellulose solubility in aqueous and nonaqueous solvents. Russian Journal of Applied Chemistry, 76, 1711–1719 (2003).
[22]. Akira I.: Chemical modification of cellulose. In ?Wood and Cellulosic Chemistry‘ (eds.: Hon D. N-S., Shiraishi N.) Marcel Dekker, New York, 599–626 (2001).
[23]. At Florence, Pu, Jani: “Novel Oral-Drug Formulations-Their Potential in Modulating Adverse-Effects” Drug Saf., 410(3), 233-266,199.
[24]. B F Goodrich Company Technical Literature: Carbopol Resin Handbook, 1991.
[25]. Handbook of Pharmaceutical Excipients, Washington DC, American Pharmaceutical Association / Pharmaceutical Society of Great Britain, 1986,41-4.
[26]. Nae H N, Reichert W W: “Rheological Properties of Lightly Cross-linked Carboxy Copolymers in Aqueous Solutions,” Rheologica Acta,31,(4), pp 351-360, 1992.
[27]. Ishikawa S et al: “Evaluation of the Rheological Properties of Various Kinds of Carboxyvinyl polymer Gels,” Chem. Pharm. Bull, 36(6), 2118-2127, 198.
[28]. hosterman, J.W. and S.H. Patterson. 1992. Bentonite and Fuller's earth resources of the United States. U.S. Geological Survey Professional Paper 1522. United States Government Printing Office, Washington D.C., USA.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









