{ DOWNLOAD AS PDF }
 ABOUT AUTHOR
ABOUT AUTHOR
Dr. (Mrs.) Anita Singh
Department of Home Science,
Kr. R.C.M. P.G College Mainpuri, U.P, India
dranitasinghkrcm@gmail.com
ABSTRACT
Human immunodeficiency virus (HIV) is a lent virus (slowly-replicating retrovirus) that causes acquired immunodeficiency syndrome (AIDs). Infection with HIV occurs by the transfer of blood, semen, vaginal fluid, pre-ejaculate, or breast milk. Within these bodily fluids, HIV is present as both free virus particles and virus within infected immune cells. HIV enters macrophages and CD4+ T cells by the adsorption of glycoproteins on its surface to receptors on the target cell followed by fusion of the viral envelope with the cell membrane and the release of the HIV capsid into the cell. There is no cure for HIV/AIDS, but a variety of drugs can be used in combination to control the virus.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2430
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 9 Received On: 01/03/2016; Accepted On: 08/04/2016; Published On: 01/09/2016 How to cite this article: Singh A; Acquired Immunodeficiency Syndrome (AIDS): A Review; PharmaTutor; 2016; 4(9); 29-32 |
INTRODUCTION
Acquired immune deficiency syndrome (AIDS) is a disease resulting from infection with human immunodeficiency virus (HIV). Since the first case of AIDS was reported in 1981 in the United States. The advent of highly active anti-retroviral therapy (HAART) significantly reduces HIV-1 infection mortality. However, HIV-1 infection cannot be controlled fully because HIV-1 can acquire resistance against all currently available antiretroviral drugs. In addition, the side-effects of anti-HIV drugs are still high [1-2]. Thus, it is still an arduous task to discover and develop new anti-HIV drugs with exact mechanism of action and novel structure type.
Human immunodeficiency virus (HIV) is a lent virus (slowly-replicating retrovirus) that causes acquired immunodeficiency syndrome (AIDs), a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive [3-4].
Pathogenesis
Infection with HIV occurs by the transfer of blood, semen, vaginal fluid, pre-ejaculate, or breast milk. Within these bodily fluids, HIV is present as both free virus particles and virus within infected immune cells. HIV is spread primarily by:
• Having multiple sex partners or the presence of other sexually transmitted diseases (STDs) can increase the risk of infection during sex.
• Sharing needles, syringes, rinse water, or other equipment used to prepare illicit drugs for injection.
• Being born to an infected mother HIV can be passed from mother to child during pregnancy, birth, or breast-feeding.
HIV infects vital cells in the human immune system such as helper T cells (specifically CD4+ T cells), macrophages, and dendritic cells. HIV infection leads to low levels of CD4+ T cells through a number of mechanisms including: Apoptosis of uninfected bystander cells, direct viral killing of infected cells, and killing of infected CD4+ T cells by CD8 cytotoxic lymphocytes that recognize infected cells. When CD4+ T cell numbers decline below a critical level, cell-mediated immunity is lost, and the body becomes progressively more susceptible to opportunistic infections [5-7].
HIV: Types
Two types of HIV have been characterized: HIV-1 and HIV-2. HIV-1 is the virus that was initially discovered and termed Human T-lymphotropic virus (HTLV-III). It is more virulent, more infective and is the cause of the majority of HIV infections globally. Because of its relatively poor capacity for transmission, HIV-2 is largely confined to West Africa.
Structure and genome of HIV
HIV is different in structure from other retroviruses. It is roughly spherical with a diameter of about 120 nm around 60 times smaller than a red blood cell. (FIG.1) It is composed of two copies of positive single-stranded RNA that codes for the virus's nine genes enclosed by a conical capsid composed of 2,000 copies of the viral protein p24. The single-stranded RNA is tightly bound to nucleocapsid proteins p7 and enzymes needed for the development of the virion are reverse transcriptase, proteases, ribonuclease and integrase.
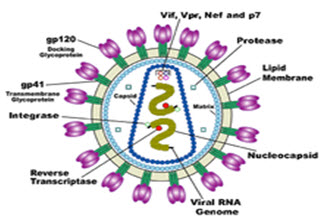
FIGURE 1:Structure and genome of HIV
A matrix composed of the viral protein p17 surrounds the capsid. This is in turn, surrounded by the viral envelope that is composed of two layers of fatty molecules called phospholipids. This protein known as Env consists of a cap made of three molecules called glycoprotein (gp) 120 and a stem consisting of three gp41 molecules that anchors the structure into the viral envelope. This glycoprotein complex enables the virus to attach and fuse with target cells to initiate the infectious cycle. Both these surface proteins especially gp120 have been considered as targets of future treatments or vaccines against HIV [8-9].
[adsense:468x15:2204050025]
THE HIV REPLICATION CYCLE
Entry to the cell
HIV enters macrophages and CD4+ T cells by the adsorption of glycoproteins on its surface to receptors on the target cell followed by fusion of the viral envelope with the cell membrane and the release of the HIV capsid into the cell.
Entry to the cell begins through interaction of the trimeric envelope complex (gp160 spike) and both CD4 and a chemokine receptor on the cell surface. Gp120 binds to integrin involved in the establishment of virological synapses, which facilitate efficient cell to cell spreading of HIV-1. The gp160 spike contains binding domains for both CD4 and chemokine receptors. Once gp120 is bound with the CD4 proteins the envelope complex undergoes a structural change exposing the chemokine binding domains of gp120 and allowing them to interact with the target chemokine receptor. This allows the N-terminal fusion peptide gp41 to penetrate the cell membrane. Repeat sequences in gp41, HR1, and HR2 then interact causing the collapse of the extracellular portion of gp41 into a hairpin. This loop structure brings the virus and cell membranes close together, allowing fusion of the membranes and subsequent entry of the viral capsid. After HIV has bound to the target cell, the HIV RNA and various enzymes, including reverse transcriptase, integrase, ribonuclease and protease is injected into the cell [10-11]. During the microtubule-based transport to the nucleus, the viral single-strand RNA genome is transcribed into double-strand DNA which is then integrated into a host chromosome.
Replication and transcription
Shortly after the viral capsid enters the cell, an enzyme called reverse transcriptase liberates the single-stranded (+) RNA genome from the attached viral proteins and copies it into a complementary DNA (cDNA) molecule. The process of reverse transcription is extremely error-prone and the resulting mutations may cause drug resistance or allow the virus to evade the body's immune system. The reverse transcriptase also has ribonuclease activity that degrades the viral RNA during the synthesis of cDNA. Together with cDNA and its complement form a double-stranded viral DNA that is then transported into the cell nucleus. The integration of the viral DNA into the host cell's genome is carried out by another viral enzyme called integrase [12].
During viral replication, the integrated DNA provirus is transcribed into mRNA which is then spliced into smaller pieces. These small pieces are exported from the nucleus into the cytoplasm. As the newly produced protein accumulates in the nucleus it binds to viral mRNAs and allows unspliced RNAs to leave the nucleus. HIV-1 and HIV-2 appear to package their RNA differently HIV-1 will bind to any appropriate RNA whereas HIV-2 will preferentially bind to the mRNA that was used to create the Gag protein itself . This may mean that HIV-1 is better able to mutate (HIV-1 infection progresses to AIDS faster than HIV-2 infection and is responsible for the majority of global infections).
Assembly and release
The final step of the viral cycle is assembly of new HIV-1 virions which begins at the plasma membrane of the host cell. The gp160 goes through the endoplasmic reticulum and is transported to the Golgi complex where it is cleaved by furin resulting in the two HIV envelope glycoproteins gp41 and gp120. These are transported to the plasma membrane of the host cell where gp41 anchors gp120 to the membrane of the infected cell. The various structural components then assemble to produce mature HIV virions. Only mature virions are then able to infect another cell, as shown in FIG. 2.

FIGURE 2: The HIV replication cycle and different classes of anti-HIVs
DIAGNOSIS OF AIDS
HIV-1 testing is initially done by an Enzyme-Linked Immunosorbent Assay (ELISA) to detect antibodies to HIV-1. Specimens with a nonreactive result from the initial ELISA are considered HIV negative unless new exposure to an infected partner or partner of unknown HIV status has occurred. Specimens with a reactive ELISA result are retested in duplicate. If the result of either duplicate test is reactive the specimen is reported as repeatedly reactive and undergoes confirmatory testing with a more specific supplemental test (e.g., Western blot or less commonly an Immuno Fluorescence Assay (IFA)) [13]. Only specimens that are repeatedly reactive by ELISA and positive by IFA or reactive by Western blot are considered HIV positive and indicative of HIV infection.
TREATMENT IMPLICATIONS
There is no cure for HIV/AIDS, but a variety of drugs can be used in combination to control the virus. Each of the classes of anti-HIV drugs blocks the virus in different ways. It's best to combine at least three drugs from two different classes to avoid creating strains of HIV that are immune to single drugs. The classes of anti-HIV drugs include:
• Non-nucleoside reverse transcriptase inhibitors (NNRTIs) - NNRTIs disable a protein needed by HIV to make copies of itself. Examples include Efavirenz (Sustiva), etravirine (Intelence) and nevirapine (Viramune).
• Nucleoside reverse transcriptase inhibitors (NRTIs) - NRTIs are faulty versions of building blocks that HIV needs to make copies of it. Examples include Abacavir (Ziagen) and the combination drugs emtricitabine and tenofovir (Truvada) and lamivudine and zidovudine (Combivir).
• Protease inhibitors (PIs) - PIs disable protease, another protein that HIV needs to make copies of it. Examples include atazanavir (Reyataz), darunavir (Prezista), fosamprenavir (Lexiva) and ritonavir (Norvir).
• Entry or fusion inhibitors - These drugs block HIV's entry into CD4 cells. Examples include enfuvirtide (Fuzeon) and maraviroc (Selzentry).
• Integrase inhibitors - Raltegravir (Isentress) works by disabling integrase, a protein that HIV uses to insert its genetic material into CD4 cells.
There are more HIV medications available now than ever before. The new generation of medications in over the past several years works better against HIV and seems to have gentler side effects than the medicines of the 1990s and resistance problem.
CONCLUSION
A lot of investigations have been targeted towards the exploration of the viral genetics of HIV-1, associated with the progression to AIDS in HIV-1 infected individuals. However, very little is known about the host genetic aspects in the pathogenesis of the infection (Michael 1999; Carrington et al. 2001; Kumar et al. 2006; Kaur and Mehra 2009). However, HIV infection cannot be controlled fully because HIV can acquire resistance against all currently available antiretroviral drugs. In addition, the side-effects of anti-HIV drugs are still high. Thus, it is still an arduous task to discover and develop new anti-HIV drugs with exact mechanism of action and novel structure type.
REFERENCES
1. Shapiro S., Guggenheim B; Quantitative Structural Activity Relationship; 1998; 17; 327.
2. Mandloi, D. Joshi, S.Khadikar, P. V. Khosla, N.; Bioorganic Medicinal Chemistry Letters; 2005; 15; 405.
3. Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ,Holmberg SD; Declining Morbidity and Mortality among Patients with Advanced Human Immunodeficiency Virus Infection; N England Journal of Medicine;1998; 338; 853–860.
4. Theodore C Pierson, Robert W Doms; HIV-1 entry inhibitors: new targets, novel therapies; Immunology Letters; 2003; 85(2); 113-118.
5. Weiss RA; How does HIV cause AIDS?; Science,; 1993;5112; 1273-1279.
6. Douek DC, Roederer M, Koup RA; Emerging Concepts in the Immunopathogenesis of AIDS, Annual Review of Medicine; 2009; 60; 471–484.
7.Gilbert PB, McKeague IW, Eisen G, Mullins C, Guéye-Ndiaye A, Mboup S, Kanki PJ; Comparison of HIV-1 and HIV-2 infectivity from a prospective cohort study in Senegal; Statistics in Medicine; 2003; 22(4); 573–593.
8. National Institute of Health Crystal structure of key HIV protein reveals new prevention, treatment targets (Press release) 1998.
9. Pope M, Haase A, Transmission; acute HIV-1 infection and the quest for strategies to prevent infection; Natural Medicine; 2003; 9(7); 847–852.
10. Haedicke, J.; Brown, C. Naghavi, M. H.; The brain-specific factor FEZ1 is a determinant of neuronal susceptibility to HIV-1 infection; Proceedings of the National Academy of Sciences; 2009; 106(33); 14040–14045.
11. Hiscott J, Kwon H, Genin P; Hostile takeovers: viral appropriation of the NF-kB pathway; Journal of Clinical Invest.; 2001; 107(2); 143–151.
12.Hallenberger S, Bosch V, Angliker H, Shaw E, Garten W; Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160; Nature; 1992; 360(6402); 358–361.
13. Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR; Identification of a major co-receptor for primary isolates of HIV-1; Nature; 1996; 381(6584); 661–666
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









