{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Sabir M. Shaikh*1, Rajendra C. Doijad1, Amol S. Shete1, Poournima S. Sankpal2
1 Department of Pharmaceutics, Shree Santkrupa College of Pharmacy, Ghogaon, Karad, Maharashtra, India.
2 Department of Pharmaceutical chemistry, Shree Santkrupa College of Pharmacy, Ghogaon, Karad, Maharashtra, India.
*sabirmshaikh17@gmail.com
ABSTRACT
For several decades pharmacist have been aware of the need to protect their products against microbial contamination but it is only during the last one or perhaps two decades the serious thought of has been applied to the science of preservation. Preservatives are commonly used as additives in pharmaceutical products, food and cosmetics. Some of the liquid preparation are susceptible to microbial contamination because of the nature of ingredients present in it. Such preparation are protected by preservatives which avoids degradation and alteration of the product.A preservative is a natural or synthetic chemical added to various products which helps to prevent microbial decomposition. Present article deals with the study of ideal properties, classification, mechanism of action, Pharmaceutical applications and its impact on health of various preservatives used in pharmaceuticals.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2410
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 5 Received On: 08/12/2015; Accepted On: 04/01/2016; Published On: 01/05/2016 How to cite this article: Shaikh SM, Doijad RC, Shete AS, Sankpal PS; A Review on: Preservatives used in Pharmaceuticals and impacts on Health; PharmaTutor; 2016; 4(5); 25-34 |
INTRODUCTION
A preservative is a natural or synthetic chemical that is added to products such as foods, pharmaceuticals, paints, biological samples, wood, etc. to prevent decomposition by microbial growth or by undesirable chemical changes. Preservatives are substances that are commonly added to various foods and pharmaceutical products in order to prolong their shelf life. The addition of preservatives to such products, especially to those that have higher water content, is essential for avoiding alteration and degradation by microorganisms during storage. Preservatives are put in foods to inhibit growth of bacteria, yeasts, or molds that can cause disease. Chemical preservation cannot totally keep products from spoiling, but they slow the spoiling process caused by microorganisms. Frozen and canned foods often do not contain any preservatives. Processed Foods are foods that are put through a process to kill harmful bacteria that may form in the food. These processes are supposed to be helpful to the products, but they can also add harmful substances.
When a natural food is processed it may be crushed, heated and have chemicals added to it that may kill all the nutritional value of the food. Then often additives are added to the product to put back some vitamins and nutrients that were lost when the food product was processed. Preservative are added to keep foods fresh and to keep them from spoiling.[1] The first preservatives to be used were vinegar, salt and sugar. Now many of the preservatives are man-made chemicals.Many of the synthetic food and cosmetic additives are considered to be safe, but some of them were found to be carcinogenic and toxic and it is better to limit their use. In general all synthetic chemical additives and preservatives may be avoided, as many of them have not been properly tested. It happens fairly often that a synthetic chemical additive that has been judged to be safe and has been used for year is later found to be toxic and in some cases causes children and adult to die.
All of the chemical food coloring can be potentially harmful, so it is better to avoid all food coloring made from synthetic chemicals. It is important to understand that the efficacy of antimicrobial preservatives relies, by definition, on their ability to kill live cells; in other words, their toxicity is an unavoidable component of their reason of being. A number of natural extracts, plants and essential oils contain substances that have the power to effectively kill bacteria, yeast and fungi; however, in many cases these substances are or can be toxic for humans, too. A typical example is citrus or grapefruit seed extracts: although these have natural antimicrobial properties, some of their constituents are thought to be responsible for life-threatening hormonal imbalances. Also, citrus seed extracts are not approved for cosmetic use in Europe and in Japan, and are therefore not an option in those countries. In the last few months, a new type of natural preservative has appeared on the market. Similar in look, feel and scent to an essential oil blend, and made by combining active fractions of essential oils, this new preservative system seems to have the potential to address the needs of those skin care manufacturers who want their products to be completely natural - yet, being such a new product, some time might be required before its efficacy and possible contraindications are proven once for all.[2]
Current review focuses on the commonly used preservatives, their chemistry, mechanism of action, factors to be considered while selecting preservatives, their potential toxicities and impact on the health caused by their repeated use.
Ideal Properties of Preservatives
1. It should not be irritant.
2. It should not be toxic.
3. It should be physically and chemically stable.
4. Preservative should be compatible with other ingredients used in formulation.
5. It should be act as good antimicrobial agent and should exert wide spectrum of activity.
6. It should act as preservative in small concentration i.e. it must be potent.
7. It should maintain activity throughout product manufacturing, shelf life and usage.[3]
CLASSIFICATION OF PRESERVATIVES
Preservatives are classified on variety of the basis and some of these are as follows
A. CLASSIFICATION BASED ON MECHANISM OF ACTION
1. Antioxidants:
The agent which prevent oxidation of Active pharmaceutical ingradient which otherwise undergo degradation due to oxidation as they are sensitive to oxygen.
Eg.Vitamin E
Vitamin C
Butylatedhydroxyanisole ( BHA).
Butylatedhydroxytoluene (BHT).
2. Antimicrobial agents:
The agent which active against gram positive & gram negative micro-organism which causes degradation of pharmaceutical preparation. Which are active in small inclusion level.
Eg. Benzoates
Sodium benzoate
Sorbates
3. Chelating agents:
The agents which form the complex with pharmaceutical ingredient and prevent the degradation of pharmaceutical formulation.
Eg. Disodium ethylenediaminetetraacetic acid (EDTA)
Polyphosphates
Citric acid
B. CLASSIFICATION BASED ON SOURCE
1. Natural Preservatives:-
These drugs are obtained by natural sources that is plant, mineral sources, animal etc.
Eg. Neem Oil
Salt (sodium chloride)
Lemon
Honey
2. Artificial Preservatives:
These preservative are man made by chemical synthesis active against by various micro-organisms in small concentration.
Eg. Benzoates
Sodium benzoate Sorbates, propionets, nitrites.[4]
MECHANISM OF ACTION: PRESERVATIVES HOW THEY ACT?
Natural substances such as salt, sugar, vinegar, and diatomaceous earthare also used as traditional preservatives. Certain processes such as freezing, pickling, smokingand saltingcan also be used to preserve food. Another group of preservatives targets enzymes in fruits and vegetables that continue to metabolize after they are cut. For instance, citricand ascorbic acidsfrom lemonor other citrusjuice can inhibit the action of the enzyme phenolase which turns surfaces of cut apples and potatoes brown. Caution must be taken, however, since FDA standards do not currently require fruit and vegetable product labels to accurately reflect the type of preservative used in the products.[5]
Antimicrobial agent:-The agent which active against gram positive & gram negative micro-organism which causes degradation of pharmaceutical preparation. Which are active in small inclusion level. Which acting by cell wall inhibition, protein synthesis inhibition, DNA &RNA synthesis inhibition.
Example- Benzoates, Sodiumbenzoate, Sorbates, Propionates, Nitrites
Antioxidants: The agent which prevent oxidation of pharmaceutical formulation. Antioxidant agents are acting self reducing agent and prevent oxidation of oxygen sensitive ingredients.
Example:-Sulfites , Vitamin E, Vitamin C, Butylatedhydroxyanisole ( BHA). ,Butylatedhydroxytoluene (BHT).
Chelating agent: Which forming a complex with pharmaceutical ingredients and prevent the degradiation of formulation.
Examples:- Disodium ethylenediamine tetraacetic acid (EDTA), Polyphosphates, Citric acid.
PRESERVATIVES:
Ethyl Alcohol
-Synonyms: Ethanolum (96 per centum); ethyl alcohol; ethyl hydroxide; grain alcohol; methyl carbinol.
-Chemical Name: Ethanol
-Empirical Formula and Molecular Weight: C2H6O 46.07
Structural Formula
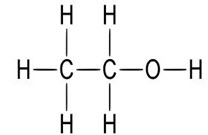
-Functional Category: Antimicrobial preservative; disinfectant; skin penetrant; solvent.
-Applications in Pharmaceutical Formulation or Technology: Ethanol and aqueous ethanol solutions of various concentrations are widely used in pharmaceutical formulations and cosmetics. Although ethanol is primarily used as a solvent, it is also employed as a disinfectant, and in solutions as an antimicrobial preservative. Topical ethanol solutions are used in the development of transdermal drug delivery systems as penetration enhancers. Ethanol has also been used in the development of transdermal preparations as a co-surfactant.
-Description
In the BP 2009, the term ‘ethanol’ used without other qualification refers to ethanol containing 599.5% v/v of C2H6O.The term ‘alcohol’, without other qualification, refers to ethanol 95.1–96.9%v/v. Where other strengths are intended, the term ‘alcohol’ or ‘ethanol’ is used, followed by the statement of the strength. In the PhEur 6.0, anhydrous ethanol contains not less than99.5% v/v of C2H6O at 208C. The term ethanol (96%) is used to describe the material containing water and 95.1–96.9% v/v of C2H6O at 208C.In the USP 32, the term ‘dehydrated alcohol’ refers to ethanol 599.5% v/v. The term ‘alcohol’ without other qualification refers to ethanol 94.9–96.0% v/v. In the JP XV, ethanol (alcohol) contains 95.1–96.9% v/v (by specific gravity) of C2H6O at 158C.In the Handbook of Pharmaceutical Excipients, the term ‘alcohol’ is used for either ethanol 95% v/v or ethanol 96% v/v. Alcohol is a clear, colorless, mobile, and volatile liquid with as light, characteristic odor and burning taste
-Typical Properties: Antimicrobial activity Ethanol is bactericidal in aqueous mixtures at concentrations between 60% and 95% v/v; the optimum concentration is generally considered to be 70% v/v. Antimicrobial activity is enhanced in the presence of eidetic acid or edentate salts. Ethanol is inactivated in the presence of nonionic surfactants and is ineffective against bacterial spores. Boiling point 78.158C Flammability Readily flammable, burning with a blue, smokeless flame. Flash point 148C (closed cup). Solubility Miscible with chloroform, ether, glycerin, and water (with rise of temperature and contraction of volume). Specific gravity 0.8119–0.8139 at 208CNote the above typical properties are for alcohol (ethanol 95% or96% v/v).
-Incompatibilities: In acidic conditions, ethanol solutions may react vigorously with oxidizing materials. Mixtures with alkali may darken in color owing to a reaction with residual amounts of aldehyde.[6]
Alpha Tocopherol
-Synonyms: Copherol F1300; 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-ol; E307; RRR-a-tocopherolum; synthetic alpha tocopherol; all-rac-a-tocopherol; dl-a-tocopherol;5,7,8-trimethyltocol.
-Chemical Name: (2RS,40RS,80RS)-2,5,7,8-Tetramethyl-2-(40,80,120-trimethyltridecyl)-6-chromanol
-Empirical Formula and Molecular Weight: C29H50O2 & 430.72
-Structure
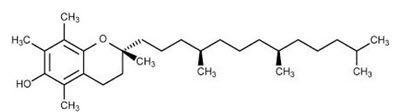
-Structural Formula
Alpha tocopherol: R1 = R2 = R3 = CH3
Beta tocopherol: R1 = R3 = CH3; R2 = H
Delta tocopherol: R1 = CH3; R2 = R3 = H
Gamma tocopherol: R1 = R2 = CH3; R3 = H
-Functional Category: Antioxidant; therapeutic agent.
-Applications in Pharmaceutical Formulation or Technology: Alpha tocopherol is primarily recognized as a source of vitamin E, and the commercially available materials and specifications reflect this purpose. While alpha tocopherol also exhibits antioxidant properties, the beta, delta, and gamma tocopherols are considered to be more effective as antioxidants. Alpha-tocopherol is a highly lipophilic compound, and is anexcellent solvent for many poorly soluble drugs of wide spread regulatory acceptability, tocopherols are of value in oil- or fat-based pharmaceutical products and are normally used in the concentration range 0.001–0.05% v/v. There is frequently an optimum concentration; thus the autoxidation of linoleic acid and methyllinolenate is reduced at low concentrations of alpha tocopherol, andis accelerated by higher concentrations. Antioxidant effectiveness can be increased by the addition of oil-soluble synergists such as lecithin and ascorbylpalmitate. Alpha tocopherol may be used as an efficient plasticizer. It hasbeen used in the development of deformable liposomes as topical formulations.
-Description: Alpha tocopherol is a natural product. The PhEur 6.0 describes alpha-tocopherol as a clear, colorless or yellowish-brown, viscous, oily liquid.
-Typical Properties: Boiling point 2358C, Density 0.947–0.951 g/cm3, Flash point 2408C, Ignition point 3408C
Refractive index n D 20 = 1.503–1.507 Solubility Practically insoluble in water; freely soluble in acetone, ethanol, ether, and vegetable oils.
11 Stability and Storage Conditions Tocopherols are oxidized slowly by atmospheric oxygen and rapidly by ferric and silver salts. Oxidation products include tocopheroxide, tocopheryl quinone, and tocopheryl hydroquinone.
-Safety: Tocopherols (vitamin E) occur in many food substances that are consumed as part of the normal diet. The daily nutritionalrequirement has not been clearly defined but is estimated to be3.0–20.0 mg. Absorption from the gastrointestinal tract is dependent upon normal pancreatic function and the presence of bile. Tocopherols are widely distributed throughout the body, with some ingested tocopherol metabolized in the liver; excretion of metabolitesis via the urine or bile. Individuals with vitamin E deficiency are usually treated by oral administration of tocopherols, although intramuscular and intravenous administration may sometimes be used.[7]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Ascorbic Acid
-Synonyms: Acidumascorbicum; C-97; cevitamic acid; 2,3-didehydro-L-threohexono- 1,4-lactone; E300; 3-oxo-L-gulofuranolactone, enol form;vitamin C.
-Chemical Name: L-(þ)-Ascorbic acid
-Empirical Formula and Molecular Weight: C6H8O6 & 176.13
-Structural Formula
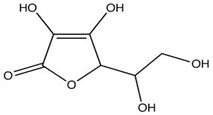
-Functional Category: Antioxidant; therapeutic agent.
-Applications in Pharmaceutical Formulation orTechnology: Ascorbic acid is used as an antioxidant in aqueous pharmaceutical formulations at a concentration of 0.01–0.1% w/v. Ascorbic acidhas been used to adjust the pH of solutions for injection, and as adjunt for oral liquids. It is also widely used in foods as an antioxidant. Ascorbic acid has also proven useful as a stabilizing agent in mixed micelles containing tetrazepam.
-Description: Ascorbic acid occurs as a white to light-yellow-colored, non hygroscopic, odorless, crystalline powder or colorless crystals with asharp, acidic taste. It gradually darkens in color upon exposure tolight.
-Stability and Storage Conditions: In powder form, ascorbic acid is relatively stable in air. In theabsence of oxygen and other oxidizing agents it is also heat stable. Ascorbic acid is unstable in solution, especially alkaline solution,readily undergoing oxidation on exposure to the air. The oxidation process is accelerated by light and heat and is catalyzed bytraces of copper and iron. Ascorbic acid solutions exhibit maximum stability at about pH 5.4. Solutions may be sterilized by filtration.
The bulk material should be stored in a well-closed nonmetallic container, protected from light, in a cool, dry place.
-Incompatibilities:Incompatible with alkalis, heavy metal ions, especially copper andiron, oxidizing materials, methenamine, phenylephrine hydrochloride, pyrilamine maleate, salicylamide, sodium nitrite, sodiumsalicylate, theobromine salicylate, and picotamide. Additionally,ascorbic acid has been found to interfere with certain colorimetric assays by reducing the intensity of the color produced.
-Safety: Ascorbic acid is an essential part of the human diet, with 40 mg being the recommended daily dose in the UK and 60 mg in the USA. However, these figures are controversial, with some advocating doses of 150 or 250mg daily. Mega doses of 10 g daily have also been suggested to prevent illness although such large doses are now generally considered to be potentially harmful. The body can absorb about 500 mg of ascorbic acid daily with any excess immediately excreted by the kidneys. Large doses may cause diarrhea or other gastrointestinal disturbances. Damage to the teeth has also been reported. However, no adverse effects have been reported at the levels employed as an antioxidant in foods, beverages, and pharmaceuticals.[8]
Sodium Benzoate
-Synonyms Benzoic acid sodium salt; benzoate of soda; E211; natriibenzoas; natriumbenzoicum; sobenate; sodiibenzoas; sodium benzoic acid.
-Chemical Name Sodium benzoate
-Empirical Formula and Molecular Weight C7H5NaO2 & 144.11
-Structural Formula
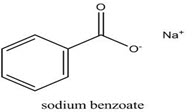
-Functional Category Antimicrobial preservative; tablet and capsule lubricant.
-Applications in Pharmaceutical Formulation or Technology
Sodium benzoate is used primarily as an antimicrobial preservative in cosmetics, foods, and pharmaceuticals. It is used in concentrations of 0.02–0.5% in oral medicines, 0.5% in parenteral products, and 0.1–0.5% in cosmetics. The usefulness of sodium benzoate as apreservative is limited by its effectiveness over a narrow pH range.
Sodium benzoate is used in preference to benzoic acid in some circumstances, owing to its greater solubility. However, in some applications it may impart an unpleasant flavor to a product. Sodium benzoate has also been used as a tablet lubricant at 2–5%w/w concentrations. Solutions of sodium benzoate have also been administered, orally or intravenously, in order to determine liver function.
-Description Sodium benzoate occurs as a white granular or crystalline, slightly hygroscopic powder. It is odorless, or with faint odor of benzoin and has an unpleasant sweet and saline taste.
-Typical Properties Acidity/alkalinity pH = 8.0 (saturated aqueous solution at 258C).It is relatively inactive above approximately pH 5.Antimicrobial activity Sodium benzoate has both bacteriostatic and antifungal properties attributed to undissociated benzoicacid;
-Incompatibilities Incompatible with quaternary compounds, gelatin, ferric salts, calcium salts, and salts of heavy metals, including silver, lead, and mercury. Preservative activity may be reduced by interactions with kaolin or nonionic surfactants. Method of Manufacture Prepared by the treatment of benzoic acid with either sodium carbonate or sodium bicarbonate.
-Safety Ingested sodium benzoate is conjugated with glycine in the liver toyield hippuric acid, which is excreted in the urine. Symptoms of systemic benzoate toxicity resemble those of salicylates. Where as oral administration of the free-acid form may cause severe gastric irritation, benzoate salts are well tolerated in large quantities: e.g. 6g of sodium benzoate in 200mL of water is administered orally as a liver function test. Clinical data have indicated that sodium benzoate can produce nonimmunological contact urtcaria and nonimmunological immediate contact reactions.[9]
Sodium Chloride
-Synonyms Alberger; chlorure de sodium; common salt; hopper salt; natriichloridum; natural halite; rock salt; saline; salt; sea salt; table salt.
-Chemical Name Sodium chloride
-Empirical Formula and Molecular Weight NaCl & 58.44
-Structural Formula
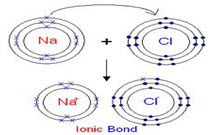
-Functional Category Tablet and capsule diluent; tonicity agent.
-Applications in Pharmaceutical Formulation or Technology Sodium chloride is widely used in a variety of parenteral and nonparenteral pharmaceutical formulations, where the primary use is to produce isotonic solutions. Sodium chloride has been used as a lubricant and diluent incapsules and direct-compression tablet formulations in the past, although this practice is no longer common. Sodium chloride has also been used as a channeling agent and as an osmotic agent in the cores of controlled-release tablets. It has been used as aporosity modifier in tablet coatings, and to control drug release from microcapsules.
-Description Sodium chloride occurs as a white crystalline powder or colorless crystals; it has a saline taste. The crystal lattice is a face-centeredcubic structure. Solid sodium chloride contains no water of crystallization although, below 08C, salt may crystallize as a dihydrate.
-Stability and Storage Conditions Aqueous sodium chloride solutions are stable but may cause the separation of glass particles from certain types of glass containers. Aqueous solutions may be sterilized by autoclaving or filtration. The solid material is stable and should be stored in a well-closed container
-Incompatibilities Aqueous sodium chloride solutions are corrosive to iron. They also react to form precipitates with silver, lead, and mercury salts. Strong oxidizing agents liberate chlorine from acidified solutions of sodium chloride. The solubility of the antimicrobial preservative methyl parabenis decreased in aqueous sodium chloride solutions and the viscosity of carbomer gels and solutions of hydroxylethylcellulose or hydroxypropyl cellulose is reduced by the addition of sodium chloride.
-Safety Sodium chloride is the most important salt in the body for maintaining the osmotic tension of blood and tissues.[10]
Sodium Sulfite
-Synonyms Disodium sulfite; exsiccated sodium sulfite; E221; natriisulfas anhydricus; sulfurous acid disodium salt.
-Chemical Name Sodium sulfite
-Empirical Formula and Molecular Weight: Na2SO3 & 126.04
-Structural Formula

-Functional Category Antimicrobial preservative; antioxidant.
-Applications in Pharmaceutical Formulation or Technology
Sodium sulfite is used as an antioxidant in applications similar to those for sodium metabisulfite. It is also an effective antimicrobial preservative, particularly against fungi at low pH (0.1% w/v of sodium sulfite is used). Sodium sulfite is used in cosmetics, food products, and pharmaceutical applications such as parenteral formulations, inhalations, oral formulations, and topical preparations.
-Description
Sodium sulfite occurs as an odorless white powder or hexagonal prisms. Note that the commercially available sodium sulfite is often presented as a white to tan- or pink-colored powder that would notconform to the pharmacopeial specification.
-Typical Properties
Acidity/alkalinity pH = 9 for an aqueous solution. Density 2.633 g/cm3 Hygroscopicity Hygroscopic. Solubility Soluble 1 in 3.2 parts of water; soluble in glycerin; practically insoluble in ethanol (95%). Stability and Storage Conditions Sodium sulfite should be stored in a well-closed container in a cool, dry, place.[11]
Methyl paraben
-Synonyms Aseptoform M; CoSept M; E218; 4-hydroxybenzoic acid methylester; metagin; Methyl Chemosept; methylisparahydroxybenzoas;methyl p-hydroxybenzoate; Methyl Parasept; Nipagin M; SolbrolM; Tegosept M; Uniphen P-23.
-Chemical Name Methyl-4-hydroxybenzoate
-Empirical Formula and Molecular Weight: C8H8O3 & 152.15
-Structural Formula
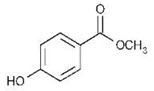
-Functional Category Antimicrobial preservative.
-Applications in Pharmaceutical Formulation or Technology
Methylparaben is widely used as an antimicrobial preservative in cosmetics, food products, and pharmaceutical formulations. It may be used either alone or in combination with other parabens or with other antimicrobial agents. In cosmetics, methylparaben is the most frequently used antimicrobial preservative. The parabens are effective over a wide pH range and have abroad spectrum of antimicrobial activity, although they are most effective against yeasts and molds. Antimicrobial activity increases as the chain length of the alkyl moiety is increased, but aqueous solubility decreases; therefore a mixture of parabens is frequently used to provide effective preservation. Preservative efficacy is also improved by the addition of propylene glycol (2–5%), or by using parabens in combination with other antimicrobial agents such asmidurea. Owing to the poor solubility of the parabens, paraben salts(particularly the sodium salt) are more frequently used in formulations. However, this raises the pH of poorly buffered formulations.
-Description
Methylparaben occurs as colorless crystals or a white crystalline powder. It is odorless or almost odorless and has a slight burning taste.
-Typical Properties
Antimicrobial activity. Methylparaben exhibits antimicrobial activity of pH 4–8. Preservative efficacy decreases with increasing pH owing to the formation of the phenolate anion. Parabens are more active against yeasts and molds than against bacteria. They are also more active against Gram positive bacteria than against Gram-negative bacteria.
-Stability and Storage Conditions
Aqueous solutions of methylparaben at pH 3–6 may be sterilized by autoclaving at 1208C for 20 minutes, without decomposition. Aqueous solutions at pH 3–6 are stable (less than 10% decomposition) for up to about 4 years at room temperature, while aqueous solutions at pH 8 or above are subject to rapid hydrolysis(10% or more after about 60 days storage at room temperature).
-Incompatibilities
The antimicrobial activity of methylparaben and other parabens is considerably reduced in the presence of nonionic surfactants, suchas polysorbate 80, as a result of micellization. However, propylene glycol (10%) has been shown to potentiate the antimicrobial activity of the parabens in the presence of nonionic surfactants and prevents the interaction between methyl paraben and polysorbate 80. Incompatibilities with other substances, such as bentonite, magnesium trisilicate, talc, tragacanth, sodium alginate, essential oils, sorbitol, and atropine, have been reported. It also reacts with various sugars and related sugar alcohols.
-Safety
Methylparaben and other parabens are widely used as antimicrobial preservatives in cosmetics and oral and topical pharmaceutical formulations. Although parabens have also been used as preservatives in injections and ophthalmic preparations, they are now generally regarded as being unsuitable for these types of formulations owing to the irritant potential of the parabens.[12]
Potassium Benzoate
-Synonyms Benzoate of potash; benzoic acid potassium salt; E212; kaliumbenzoat; potassium salt trihydrate; ProBenz PG.
-Chemical Name Potassium benzoate
-Empirical Formula and Molecular Weight C7H5KO2 & 160.2
-Structural Formula
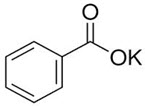
-Functional Category
Antimicrobial preservative; tablet and capsule lubricant.
-Applications in Pharmaceutical Formulation or Technology
Potassium benzoate is predominantly used as an antimicrobial preservative in a wide range of beverages, foods and some pharmaceutical formulations. Preservative efficacy increases with decreasing pH; it is most effective at pH 4.5 or below. However, atlow pH undissociated benzoic acid may produce a slight though discernible taste in food products. Increasingly, potassium benzoate is used as an alternative to sodium benzoate in applications where a low sodium content is desirable. Therapeutically, potassium benzoate has also been used in the management of hypokalemia.
-Description
Potassium benzoate occurs as a slightly hygroscopic, white, odorlessor nearly odorless crystalline powder or granules. Aqueous solutions are slightly alkaline and have a sweetish astringent taste.
-Typical Properties
Acidity/alkalinity Aqueous solutions are slightly alkaline. Melting point >3008C
Solubility. Specific gravity 1.5.
-Incompatibilities
Potassium benzoate is incompatible with strong acids and strong oxidizing agents.
-Safety
Potassium benzoate is widely used in food products and is generally regarded as a nontoxic and nonirritant material. However, people with a history of allergies may show allergic reactions when exposed to potassium benzoate. Ingestion is inadvisable for asthmatics.
Higher concentrations of potassium benzoate have been reported to cause irritation to mucous membranes. The WHO acceptable daily intake of total benzoates including potassium benzoate, calculated as benzoic acid, has been estimatedat up to 5 mg/kg of body-weight.[13]
Applications of preservatives:
Cosmetic products become easily contaminated by microbes. Containing water, oils, peptides, and carbohydrates cosmetics are a very good medium for growth of microbes. All these factors contribute to the fact that cosmetic products need very good preservation to prevent microbial growth and spoiling of the cosmetic product and also infection of the skin. Preservatives are active ingredients able to prevent the growth bacteria, fungi, & viruses.
All our preservatives have potent antimicrobial properties preventing personal care and makeup products effectively from spoiling and prolonging substantially the shelf-life. Some of these agents also have stabilizing effects able to preserve the function of various active ingredients including anti-oxidants (vitamins), emulsifiers and surfactants. The addition of such kind of stabilizers makes sure that creams, lotions and other complex cosmetic products do not separate or otherwise disintegrate.[14]
We should not have to compromise with the quality of our Natural Cosmetics by using harsh preservatives. Use the right preservative and adding the right amount to the Natural Cosmetics without loosing the mild and gentle effects of the Natural Ingredients present in the Cosmetics. In order to keep the product safe use of the right Preservative is needed.[15]
· Many natural substances offer some antibacterial benefits. Certain essential oils, like Tea Tree, Thyme and Oregano at high concentrations can be helpful with some strains of bacteria. Unfortunately, your bathroom, purse, car, or desk drawer is not an ideal condition natural cosmetics.
· Steam, heat, direct sunlight and other adverse conditions help encourage bacterial growth and most "natural preservatives" can't be used in strong enough concentrations to fight contamination without running the risk of skin irritation or allergic reactions. Others are useful only against certain strains of contaminants and for limited amounts of time.
· While Vitamin E, Neem and Rosemary Oleoresin Extract (ROE) work wonders at keeping oils from turning rancid, they don't protect against all forms of gram-positive, gram-negative bacteria and yeast which are common in unprotected cosmetic products.[16]
CONCLUSION
As we have seen that compared to synthetic preservatives which are used in preserving foods, cosmetics, etc, natural preservatives has fewer side effects. Natural preservatives, firstly is a traditional method of preserving food which have been used for centuries. Natural preservatives has been economical, and better availability, so we can easily use it. Natural preservatives not only reduce the bacterial growth but increases the shelf life of the ingredient in which it is added. It also allows them to remain fresh or maintain its consistency for a long span of time and causes no toxic effect.
Synthetic preservatives are also good but research has reported that they cause many health problems as almost all are carcinogenic in nature. Hence these must be used considering maximum safety limit for the preservatives used in pharmaceuticals as well as cosmetics.
Since there are many effective and potential uses of using natural preservatives in different ingredients, satisfactory evidence of its effectiveness and safety is still lacking. The beauty brands that have used natural preservative systems seem to have done so successfully. However, the challenges going forward lie in market challenges—lack of development in the area of new and better natural preservative systems. “The performance and cost of these systems are still not equivalent to the traditional preservatives, so they are not widely used in marketed products,”
Hence, it may better to use natural preservatives instead of synthetic one, as it provides us so many good effects.
REFERENCES
1. Chiori CO, Ghobashy AA. A potentiating effect of EDTA on thebactericidal activity of lower concentrations of ethanol. Int J Pharm ;1983; 17: 121–128.
2. European Directorate for the Quality of Medicines and Healthcare(EDQM). European Pharmacopoeia – State of Work of International Harmonisation. Pharmeuropa 2009; https://www.edqm.eu/site/-614.html (accessed 3 February 2009). 21(1): 142–143.
3. PB Nielsena, A Müllertzb, T Norlinga, HG Kristensen, The effect of a-tocopherol on the in vitro solubilisationof lipophilic drugs; Int J Pharm; 2001; 222(2); 217–224.
4. Constantinides PP et al. Tocol emulsions for drug solubilization and parenteral delivery. Adv Drug Delivery 2004; 56(9): 1243–1255.
5. Hammad MA, Muller BW, Solubility and stability of tetrazepam inmixed micelles. Eur J Pharm Sci; 1998; 7(1); 49–55.
6. Hajratwala BR. Stability of ascorbic acid. STP Pharma; 1985; 1; 281–286
7. Saleh SI, P. Wehrlé, A. Stamm, Improvement of lubrication capacity of sodium benzoate:effects of milling and spray drying. Int J Pharm 1988; 48(3); 149–157.
8. Clarke CD, Armstrong NA, Influence of pH on the adsorption ofbenzoic acid by kaolin. Pharm J; 1972; 62(3); 44–45.
9. Leigh S, J carless, B Burt, Compression characteristics of some pharmaceutical materials; J Pharm Sci; 1967; 56(7); 888–892.
10. Rees JE, Shotton E. Some observations on the ageing of sodium chloridecompacts. J Pharm Pharmacol; 1970; 22(S1); 17S–23S.
11. Islam MS, Asker AF, Photoprotection of daunorubicin hydrochloride withsodium sulfite, PDA J Pharm SciTechnol; 1995; 49(3); 122–126.
12. Nair B, Elmore AR. Final report on the safety assessment of sodium sulfite, potassium sulfite, ammonium sulfite, sodium bisulfite, ammonium bisulfite, sodium metabisulfite and potassium metabisulfite. Int JToxicol; 2003; 22(2); 63–88.
13. Decker RL, Wenninger JA. Frequency of preservative use in cosmeticformulas as disclosed to FDA—1987. Cosmet Toilet 1987; 102(12); 21–24.
14. Prickett PS, Murray HL, Mercer NH, Potentiation of preservatives (parabens) in pharmaceuticalformulations by low concentrations of propylene glycol. JPharm Sci; 1961; 50: 316–320.
15. FAO/WHO. Toxicological evaluation of certain food additives with areview of general principles and of specifications. Seventeenth report ofthe joint FAO/WHO expert committee on food additives.World Health Organ Tech Rep Ser 1974; No. 539.
16. FAO/WHO. Evaluation of certain food additives and contaminants.Twenty-seventh report of the joint FAO/WHO expert committee onfood additives. World Health Organ Tech Rep Ser 1983; No. 696.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









